Aksetin
Application instruction:
 Aksetin – generation cephalosporin II, the antibiotic of a broad spectrum of activity having antimicrobic and bactericidal effect.
Aksetin – generation cephalosporin II, the antibiotic of a broad spectrum of activity having antimicrobic and bactericidal effect.
Form of release and structure
Dosage form – powder for preparation of solution for intravenous and intramuscular administration: from white till light yellow color (in glass bottles, 1 bottle in a pack of cardboard or 100 bottles in a box cardboard (for hospitals)).
Active agent: tsefuroksy (in the form of sodium salt) in 1 bottle – 750 or 1500 mg.
Indications to use
Aksetin use for therapy of the following infectious and inflammatory diseases caused by microorganisms, sensitive to a tsefuroksim:
- Respiratory tracts: pneumonia, bronchitis, pleura empyema, lung abscess;
- ENT organs: otitis, pharyngitis, tonsillitis, sinusitis;
- Skin and soft tissues: pyoderma, ugly face, impetigo, phlegmon, furunculosis, contaminated wounds;
- Urinary tract: cystitis, chronic and acute pyelonephritis, gonorrhea, symptomatic bacteriuria;
- Bodies of a small pelvis: cervicitis, endometritis, adnexitis;
- Bones and joints: osteomyelitis and septic arthritis;
- Other infections: sepsis and meningitis, peritonitis, borreliosis (Lyme's disease).
Use of drug for prevention of infectious complications at surgical interventions on bodies of a basin, an abdominal cavity, thorax, joints is recommended, including at gullet, heart, lungs operations, at orthopedic operations, and also in vascular surgery with high risk of infectious complications.
Contraindications
Aksetin is strictly contraindicated only at hypersensitivity to his components, other cephalosporins, karbapenema or penicillin.
Drug should be used with care in the following cases:
- Exhaustion and weakening of patients;
- Diseases and bleedings of digestive tract (including nonspecific ulcer colitis), including in the anamnesis;
- Chronic renal failure;
- The period of a neonatality and prematurity at children;
- Period of pregnancy and lactation.
Route of administration and dosage
The solution prepared from powder enter intravenously (in/in) or intramusculary (in oil).
The adult usually appoint 750 mg 3 times a day, at the heavy course of an infectious disease the dose is increased up to 1500 mg by 3-4 times a day (an interval if necessary reduce till 6 o'clock). The average daily dose makes 3000-6000 mg.
To children 3 years usually are more senior appoint on 30-100 mg/kg/days in 3-4 receptions. The daily dose of 60 mg/kg in most cases is optimum.
To newborns and children up to 3 years – on 30 kg/kg/days in 2-3 receptions.
The recommended dosing modes:
- Gonorrhea: 1500 mg in oil once or on 750 mg 2 times with introduction to different areas (for example, both gluteuses);
- Bacterial meningitis: in/in, adults – on 3000 mg each 8 hours, children – on 150-250 mg/kg/days in 3-4 receptions, newborns – on 100 mg/kg/days;
- Pneumonia: in/in or 2-3 times, in oil on 1500 mg, a day within the first 2-3 days, then transfer the patient to the peroral drug containing tsefuroksy – on 500 mg 2 times a day on an extent of 7-10 days;
- Exacerbation of chronic bronchitis: in/in or 2-3 times, in oil on 750 mg, a day within the first 2-3 days, then transfer the patient to the peroral drug containing tsefuroksy – on 500 mg 2 times a day on an extent of 5-10 days;
- At lung, heart operations, vessels, a gullet: at anesthesia induction – 1500 mg in/in, then – 3 times/days, in oil on 750 mg, within 1-2 days;
- At orthopedic operations and operations on bodies of a basin, an abdominal cavity: at anesthesia induction – 1500 mg in/in, then – in oil on 750 mg in 8 and 16 hours after surgical intervention;
- At full replacement of a joint: 1500 mg of powder in a dry form mix with each package of polymer methyl - methacrylate cement before addition of liquid monomer.
At a chronic renal failure the dose is adjusted depending on the clearance of creatinine (CC): KK of 10-20 ml/minute – on 750 mg 2 times a day, KK less than 10 ml/minute – on 750 mg of 1 times a day.
To patients who are on a continuous hemodialysis using the arteriovenous shunt or on haemo filtering of high speed in intensive care unit appoint 750 mg 2 times a day. To the patients who are on haemo filtering of low speed appoint doses which apply at a renal failure.
Side effects
- From the alimentary system: ulcerations of a mucous membrane of an oral cavity, a glossitis, oral cavity candidiasis, spasms and an abdominal pain, a meteorism, vomiting or a lock, diarrhea, nausea, a pseudomembranous coloenteritis, a cholestasia, increase in activity of liver enzymes in plasma (aspartate aminotransferases, alaninaminotranspherases, an alkaline phosphatase, a lactate dehydrogenase, bilirubin);
- From system of a hemopoiesis: anemia (aplastic or hemolitic), thrombocytopenia, agranulocytosis, prothrombinopenia, eosinophilia, leukopenia, neutropenia, decrease in hemoglobin and hematocrit, lengthening of a prothrombin time;
- From an urinary system: a dysuria, a renal failure (increase in residual nitrogen of urea and creatinine in blood, decrease in clearance of creatinine);
- From a reproductive system: a vaginitis, an itch in a crotch;
- Allergic reactions: itch, rash, urticaria, fever; seldom – a bronchospasm, an acute anaphylaxis, Stephens-Johnson's syndrome, a multiformny erythema;
- Local reactions: pain, infiltrate and irritation in an injection site, phlebitis.
Special instructions
At patients in whose anamnesis allergic reactions to penicillin were noted hypersensitivity to tsefalosporinovy antibiotics is possible.
During treatment it is necessary to control functions of kidneys, especially at patients who receive Aksetin in high doses. At the infections caused by Streptococcus pyogenes, treatment should be continued within 48-72 hours after disappearance of symptoms of a disease. The recommended therapy duration – not less than 10 days.
If during treatment tsefuroksimy there is a need to determine glucose level in blood, it is necessary to apply tests with a hexokinase or glucose oxydas.
At children during therapy of meningitis decrease in hearing is possible.
At use of a tsefuroksim receiving false positive reaction of urine to glucose and false positive forward reaction of Koombs is possible.
At transfer of the patient with parenteral administration Aksetin into administration of drug containing tsefuroksy inside it is necessary to consider the general condition of the patient, sensitivity of microorganisms and weight of an infection. If in 3 days after reception of means inside clinical improvement is not noted, it is necessary to continue parenteral administration.
During treatment it is not necessary to take alcoholic beverages.
Medicinal interaction
Tsefuroksim pharmaceutical is compatible to the following means: xylitol, азлоциллин, the metronidazole, aqueous solutions containing to 1% of lidocaine of a hydrochloride, 5% solution of a dextrose, 0,9% solution of sodium of chloride, 4% solution of a dextrose and 0,18% solution of sodium of chloride, 5% solution of a dextrose and 0,45% solution of sodium of chloride, 10% solution of a dextrose, 5% solution of a dextrose and 0,225% chloride sodium solution, Ringer's solution, Hartman's solution, solution of sodium lactate, 5% solution of a dextrose and a hydrocortisone, 10% the inverted sugar in water for injections, potassium chloride (10 ¼Ý¬ó/l and 40 ¼Ý¬ó/l) in 0,9% chloride sodium solution, heparin (10 units/ml and 50 units/ml) in 0,9% chloride sodium solution.
Tsefuroksim pharmaceutical is incompatible with solution of sodium of bicarbonate of 2,74% and aminoglycosides.
The drugs reducing acidity of a gastric juice reduce absorption and bioavailability of a tsefuroksim.
At simultaneous use of aminoglycosides and diuretics the risk of emergence of nephrotoxic effects increases.
At simultaneous use of peroral loopback diuretics concentration in plasma increases and the elimination half-life of a tsefuroksim increases, there is a delay of canalicular secretion, the renal clearance decreases.
Terms and storage conditions
To store in the unavailable to children, protected from light, dry place at a temperature up to 25 ºС.
Period of validity – 2 years.
The solution prepared from powder is allowed to be stored within 48 hours in the refrigerator and within 7 hours – at the room temperature. For storage time solution can turn yellow, it is not a contraindication to its use.
The most rare disease – a disease the Kura. Only representatives of the tribe Faure in New Guinea are ill it. The patient dies of laughter. It is considered that eating of a human brain is an origin of a disease.

The unpleasant feelings connected with spring breakdown are familiar almost to each of us. Often happens that in March-April on the person...
Section: Articles about health
Climax - process of fading of reproductive function of an organism in process of its aging. At women the main sign of its approach is the termination of a menstrual cycle. Officially the menopause is diagnosed when periods are not observed in течен...
Section: Articles about health
Each person has easy indispositions which he transfers "standing", trying not to ask for medical care. Arguments at the same time are adduced same: "it is a trifle, itself will pass", "I have too many important issues", "there are no wish to spend time for doctors", etc. At good shape of health, normal working capacity and lack of suspiciousness dislike for complaints to such problems is quite natural. It is not the most correct, but very widespread type of behavior. I am glad...
Section: Articles about health
Iodine - one of thirty most important microelements in our organism. The main role of iodine consists in synthesis thyroid гормо...
Section: Articles about health
The technique of acupuncture (acupuncture) is used in the medical purposes more than three and a half millennia. It is eurysynusic and recognized as official medicine in the majority of the developed countries of the world. Influence by fine needles on so-called points...
Section: Articles about health
Scientists always aimed to offer fundamental explanations for medical problems. Their theories formed the basis of modern methods of treatment of the hardest pathologies and helped to save a set of lives. However stories are known also such theoretical constructions, following to which brought to mankind of a trouble and torture, ruined destinies and health of many people....
Section: Articles about health
On health of the person physicians know about salutary action of animals long ago. About 7 thousand years ago great Hippocrates рекоменд...
Section: Articles about health
Almost each of us during life faced dissatisfaction with own body. At such moments, as a rule, we begin to shame ourselves, urgently we go on the most rigid diet promising minus of 10 kg in a week, or we exhaust ourselves in the gym to полусм...
Section: Articles about health
The state of health of the person in many respects depends on food. The organism will well function if during food it receive only useful substances, necessary vitamins and microelements. In this case there will be no problems with digestion, with excess weight, and intellectual and physical activity will remain at the high level....
Section: Articles about health
Eyes – unique body on the structure thanks to which the person obtains about 80% of information on the world around: about a form...
Section: Articles about health
Visit of doctors – business not the most pleasant, and many people do not hurry to undergo necessary planned inspections. Such behavior is extremely thoughtless and improvident. Our health is necessary not only to us: wellbeing of darlings, children, grandsons and престар...
Section: Articles about health
What woman does not dream of a beautiful and thick hair? While physicians developed difficult schemes on hair transplant, in the industry of hairdresser's art a few years ago there was a sensation – methods of hair extension appeared. It would seem, dreams came true: though the procedure of building also does not belong to the category cheap, practically any woman can increase several times the volume of hair, change their length and color – generally, to become the real beauty queen....
Section: Articles about health
Transfusion of donor blood has almost century history. In spite of the fact that this procedure is quite usual for many people, itself п...
Section: Articles about health
Partial and the more so full loss of hearing significantly reduces quality of life. Difficulties with communication lead to loneliness and isolation. The person who badly hears experiences difficulties with social and professional implementation, quite often has problems in...
Section: Articles about health
Heart disease and blood vessels lead to disturbance of blood supply of bodies and fabrics that involves failures in their work, deterioration in health of the person, decrease in its working capacity and standard of living. Annually more than 17 million inhabitants of our planet perish from pathologies such....
Section: Articles about health
Epilepsy is one of widespread neurologic diseases. To parents, whose children suffer from this illness, it is necessary...
Section: Articles about health
History of use of an anesthesia during operations contains more than 160 years. Annually in the world hundreds of thousands of surgical interventions during which to patients the substances immersing them in a dream and saving from pain are entered are carried out. Using an anesthesia to these...
Section: Articles about health
The immunity role in growth of the child is invaluable. The proteins-immunoglobulins produced by immune system preserve the child against the diseases capable − owing to an organism weak still − to serve as a stressful factor, to become the reason of many complications and delays in development of the kid. If the immune system weakened, health of the child is under direct threat and needs active actions for strengthening of protective forces of an organism − preferably non-drug....
Section: Articles about health
Cold, puffiness of a nose, itch, the watering eyes - characteristic symptoms of the allergic rhinitis resulting from hit and...
Section: Articles about health
The words "disease" and "patient" not without reason come from one root – "pain". As a rule, symptoms of illnesses thoroughly spoil to patients life. However from this rule there are exceptions. Some diseases are shown by signs which can cause even полож...
Section: Articles about health
Tuberculosis – a serious infectious disease which development is caused by mycobacteria (Koch's bacilli). The illness is known from an extreme antiquity. Long time fight against it was considered as ineffective. Quite often the disease affected the whole families, and mortality from it was very high. It became the reason of emergence of a set of delusions concerning transmissibility and a possibility of treatment of tuberculosis....
Section: Articles about health
Long time antibiotics were considered as a panacea from all diseases and were appointed even at insignificant symptoms of an infection. Even now...
Section: Articles about health
Statistically, at the address to doctors seven of each ten patients complain of a headache. Actually it is much more people who are periodically feeling unpleasant feelings such. Many people, apart from a headache the reason for serious fear...
Section: Articles about health
Very often as a source of the infection which caused a disease serves our house - the place which a priori has to be safe. However disease-producing bacteria can perfectly feel not only in insanitary conditions, but also in our apartment if not to carry out due care of favourite places of their dwelling. What they − sources of their reproduction? Let's consider 10 most widespread places in our house, the most dangerous from the point of view of infection with microorganisms....
Section: Articles about health
With age in a human body harmful substances collect. We receive them with food and water, at inhalation contaminated air...
Section: Articles about health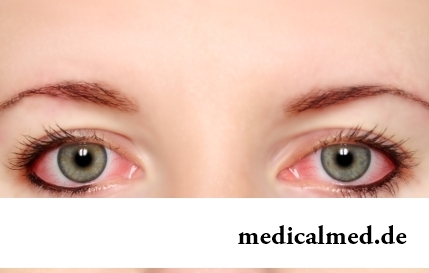
The sclera and mucous membrane of an eye are intensively supplied with blood vessels which problem - to saturate nervous tissues of body with nutrients and oxygen. In a normality vessels are almost not noticeable, however at their expansion (owing to истонч...
Section: Articles about health
The trophic ulcer is not an independent disease. This heavy complication arising owing to a thermal injury (a burn or a frostbite), chronic pathologies of arteries or veins of the lower extremities, a diabetes mellitus, and also some defeats of connecting fabric, absorbent vessels, skin or nervous trunks. Pathology is shown in the form of not healing wound located on the internal surface of a shin, a foot sole, a heel or toes....
Section: Articles about health