Anastrozol
Application instruction:
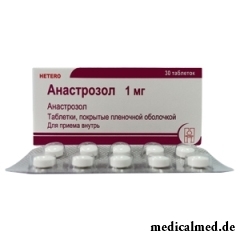 Anastrozol – drug with antineoplastic action.
Anastrozol – drug with antineoplastic action.
Form of release and structure
Dosage form of release of Anastrozol – a tablet, film coated: round, biconvex, almost white or white; on a break two layers – white or almost white color a kernel and a film cover (on 7, 10, 14 or 30 pieces in blisters or blister strip packagings, till 1-10 blisters/packagings in a cardboard pack; on 7, 10, 14, 20, 28, 30, 40, 50, 60, 100 or 120 pieces in banks, after 1 bank in a cardboard pack).
Structure of 1 tablet:
- active ingredient: анастрозол – 1 mg;
- auxiliary components: sodium carboxymethylstarch – 2 mg, microcrystallic cellulose – 15,6 mg, monohydrate of lactose (milk sugar) – 72 mg, magnesium stearate – 0,9 mg, polyvinylpirrolidone (povidone) – 3 mg, colloid silicon dioxide – 0,5 mg;
- cover: titanium dioxide – 0,9 mg, a macrogoal of 4000 - 0,45 mg, a gipromelloza – 1,65 mg.
Indications to use
- early gormonopolozhitelny breast cancer at women in the period of a postmenopause (adjuvant therapy);
- early gormonopolozhitelny breast cancer at women in the period of a postmenopause after use for 2-3 years of Tamoxifenum (adjuvant therapy);
- widespread breast cancer at women in the period of a postmenopause (treatment).
Contraindications
Absolute:
- premenopauzalny period;
- liver failure in average/heavy degree (the safety profile for this group of patients is not studied);
- renal failure in heavy degree (at clearance of creatinine <20 ml/min.);
- the combined use with Tamoxifenum or the medicines containing estrogen;
- pregnancy and period of feeding by a breast;
- age up to 18 years (the safety profile for this group of patients is not studied);
- hypersensitivity to drug components.
Relative (Anastrozol appoint with care in the presence of the following diseases / states):
- coronary heart disease;
- hypercholesterolemia;
- osteoporosis;
- functional disturbances of a liver;
- intolerance lactose/insufficiency of lactase / глюкозо-галактозная malabsorption (lactose is a part of drug).
Route of administration and dosage
Anastrozol accept inside, it is more preferable – at the same time. Tablets need to be swallowed entirely and to wash down with water. Meal does not influence efficiency of drug.
Anastrozol appoint on 1 tablet a day. Long therapy is shown. Drug is cancelled in cases of emergence of signs of progressing of a disease. The recommended duration of adjuvant therapy – 5 years.
Dose adjustment at easy/average degree of renal and easy degree of a liver failure is not required.
Side effects
- respiratory system, bodies of a thorax and mediastinum: bronchitis, pharyngitis, sinusitis, short wind;
- lymphatic system and blood: limfedema, anemia, venous thrombembolia;
- urinogenital system: dryness of a mucous membrane of a vagina, a vulvovaginitis, infections of urinary tract, vulval bleedings (as a rule, for the first weeks after cancellation or when changing of the previous hormonal treatment on Anastrozol);
- nervous system: drowsiness, a headache, paresthesia, a depression, dizziness, sleeplessness, concern, a syndrome of a carpal tunnel (as a rule, it was observed with risk factors of a course of a disease);
- cardiovascular system: increase in arterial pressure, rushes of blood to the person;
- skeletal and muscular system and connecting fabrics: arthralgia/constraint of joints, arthritis, trigger finger, muscle/bones pain;
- alimentary system: nausea, dyspepsia, diarrhea, lock, vomiting, gastrointestinal frustration;
- gepatobiliarny system: hepatitis, increase in activity of an alkaline phosphatase, aspartate aminotransferase, alaninaminotranspherase, increase in concentration of bilirubin and activity gamma глутамилтрансферазы;
- skin and hypodermic fabrics: thinning of hair, skin rash, alopecia, sweating;
- organ of sight: cataract;
- metabolism and food: anorexia, increase in body weight, a hypercholesterolemia, a hypercalcemia (with/without increase in concentration of parathormone); decrease in mineral density of a bone tissue because of decrease in concentration of the circulating oestradiol is possible that increases risk of development of fractures of bones and osteoporosis;
- allergic reactions: a small tortoiseshell, anaphylactoid reaction, a multiformny erythema, Stephens-Johnson's syndrome, a skin vasculitis (including separate cases of a purpura), a Quincke's disease;
- others: consecutive infections, adynamy, spin/stomach/breast pain, new growths, grippopodobny syndrome.
Special instructions
Anastrozol's efficiency at a retseptorootritsatelny tumor to estrogen was not shown, except for cases when there is a previous affirmative clinical answer on Tamoxifenum. In the presence of doubts in the hormonal status of the patient the menopause needs to be confirmed with definition of sex hormones in blood serum.
The combined use with the drugs containing estrogen is contraindicated (as they level Anastrozol's action).
At preservation during therapy of uterine bleeding it is necessary to consult at the gynecologist.
At osteoporosis or existence of the increased risk of development of osteoporosis in an initiation of treatment and regularly on its extent the mineral density of a bone tissue needs to be estimated by a densitometry method, for example, a two-power x-ray absorbtsiometriya (DEXA scanning). In cases of need it is necessary to appoint therapy or prevention of osteoporosis, and also to make careful observation of a condition of the patient. Anastrozol lowers concentration of the circulating oestradiol that can lead to reduction of mineral density of a bone tissue. At the moment there are no sufficient data of rather positive action of bisfosfonat on the loss of mineral density of a bone tissue connected with Anastrozol's reception or efficiency of their use for prevention.
Data on the combined Anastrozol's use with drugs analogs of the luteinizing hormone of rileasing-hormone (LHRH) are absent.
Efficiency of the combined use with chemotherapy is not established.
Data on safety of prolonged treatment by Anastrozol are not obtained.
At Anastrozol's use more often than at therapy by Tamoxifenum, development of ischemic diseases was noted, however the statistical importance at the same time was not noted.
As during Anastrozol's use development of such disturbances as dizziness, drowsiness, an adynamy, a headache is possible, it can have an adverse effect on ability to control of motor transport and mechanisms, and also performance of other works demanding the increased concentration of attention and bystry psychomotor reactions (respect for care is necessary).
Medicinal interaction
Clinically significant medicinal interaction of Anastrozol with other often appointed drugs is absent.
The medicines containing estrogen reduce pharmacological action of Anastrozol (the combination is contraindicated).
The combined use with Tamoxifenum is not recommended (because of probability of weakening of pharmacological action of an anastrozol).
Terms and storage conditions
To store in protected from light, the place, unavailable to children, at a temperature up to 25 °C.
Period of validity – 3 years.
Name of drug
Price
Drugstore
The 74-year-old resident of Australia James Harrison became blood donor about 1000 times. It has a rare blood group which antibodies help to survive the newborn with a severe form of anemia. Thus, the Australian saved about two million children.

Reactive pancreatitis - the disease which is characterized by inflammatory process in a pancreas which arises more often everything...
Section: Articles about health
Several decades ago the basil (the district khan, реан, Reagan) was considered as a part of the Caucasian or east cuisine, but today it strongly took the place on tables of Russians. Greens of this plant possess a strong, pleasant smell and specific fresh taste, because of to...
Section: Articles about health
There is an opinion that at low temperatures safety of products is ensured longer and better thanks to what the refrigerator is considered the most suitable place for storage of food. In most cases it is fair, however there is a number of products for which low temperatures – the main reason of their premature damage. Storage in the refrigerator leads to their bystry rotting, emergence of a mold, is followed by loss of vitamins and tastes. What products it is better to remove...
Section: Articles about health
For anybody not a secret that our country is one of the most "drinking" in the world. At clear understanding of that the use of strong...
Section: Articles about health
Frosty air, fresh wind and easy snowball at most of Russians are associated with cheerfulness, health and cheerful entertainments on which our winter is so generous. But, unfortunately, cold season sometimes brings also troubles with health. It is not about a season...
Section: Articles about health
The drugs stopping or oppressing life activity of pathogenic microorganisms are widely applied in clinical practice from 40th years of the last century. Originally antibiotics were called only substances natural (animal, vegetable or microbic) origins, but over time this concept extended, and it includes also semi-synthetic and completely artificial antibacterial drugs....
Section: Articles about health
Life of the modern child is extremely active and difficult. Information strain which is experienced by the school student and did not dream the pupil...
Section: Articles about health
Stability of a hormonal background is one of the most important conditions of preservation of health of the woman. At the same time endocrine system – the thin device extremely sensitive to any external influences. Changes of an image жиз can become the reason of hormonal failure...
Section: Articles about health
Aspirin (acetylsalicylic acid) – one of those drugs which are known literally to all. It is available in each home first-aid kit, and many accept it at the first signs of an indisposition, often without having a fair idea of properties and therapeutic effect of drug. Meanwhile, impact of aspirin on a human body is very various, and is not always favorable. About it it is important to foreknow, in order to avoid emergence of problems with health....
Section: Articles about health
Climax - process of fading of reproductive function of an organism in process of its aging. At women the main sign of its approach showing...
Section: Articles about health
History of mankind contains several tens of epidemics whose emergence was compared by eyewitnesses and historians to doomsday. The most terrible of them claimed the lives of millions of people, having made even the whole people to the person of the earth. What they − the diseases striking terror? Daringly...
Section: Articles about health
White teeth and the Hollywood smile – a dream of many people. Long time was considered that the plaque on teeth and change of their color – destiny of those who incorrectly eat smokes and badly brushes teeth. But the paradox is available: at everything the variety of toothpastes, brushes existing today for toothbrushing and conditioners for a mouth the number of the people hesitating of a plaque on teeth does not decrease. Moreover, the plaque is formed even at small children who definitely do not smoke and have no coffee. So in what business, and опас...
Section: Articles about health
According to doctors, more than a half of men of 25-50 years suffer from frustration of the urinogenital sphere, but sees a doctor from them меньшинс...
Section: Articles about health
The state of health of the person depends on many factors. One of the most important is the constant, but not exhausting a physical activity. In the presence of various illnesses specialists often advise patients to do swimming which by right borrows ведущ...
Section: Articles about health
Weakness of an ankle joint – very widespread problem. Its existence is demonstrated by tendency to a podvorachivaniye of legs when walking on heels, frequent painful sprains, pain on average and anonymous toes even after small loadings. Usually people with such pathology take off unpleasant effects by means of the anesthetizing pulverizing and ointments, but it does not lead to radical elimination of a problem. Meanwhile, at the known persistence it is possible to strengthen an ankle to the house...
Section: Articles about health
All diseases from nerves – in this joke a big element of truth, are said by doctors. Constant stresses lead body to decrease in protective forces...
Section: Articles about health
No, probably, the person who would not have cold. Cold, cough, a headache – these symptoms are known to everyone. The peak of catarrhal diseases is the share of fall. SARS already came to schools and kindergartens, flu slowly makes the way to the cities, in a word, з...
Section: Articles about health
The pancreas performs two functions in a human body: release of enzymes without which digestion of carbohydrates, fats and proteins, and a producing hormones is impossible. The most important of them - insulin, is the main participant of carbohydrate metabolism normalizing processes of education and utilization of glucose, the main energy source for an organism....
Section: Articles about health
History of use of an anesthesia during operations contains more than 160 years. Annually in the world hundreds of thousands surgical вм are carried out...
Section: Articles about health
Diseases of joints often begin imperceptibly for the person. The first stages of destruction of the cartilaginous tissue providing soft and free sliding of heads of bones in joint bags proceed slowly and absolutely without serious consequences. Especially unpleasantly for that this пр...
Section: Articles about health
The hysteromyoma is diagnosed more than at a third of women 35 years are more senior. This high-quality new growth which at early stages successfully resolves by means of medicines. It is necessary to resort to an operative measure only when patients too late address specialists, or therapeutic methods do not give the expected effect because of specific features of an organism. Besides, there is a large number of quite effective national...
Section: Articles about health
Ayurveda - the most ancient tselitelsky practice which came to us from India. It represents the doctrine about maintenance physical, ps...
Section: Articles about health
80% of women at least once to lives complained of discomfortable feelings to breasts, consolidations and nagrubaniye. These are mastopathy symptoms. The mastopathy is characterized by change of a ratio between ferruterous and connective tissue tissues of mammary glands. It can bring...
Section: Articles about health
To look healthy and means well-groomed not only to be pleasant to people around, but also to feel strong, sure and taken place. Specialists in the field of cosmetology quite often note that not all women are able to look after face skin. Many women incorrectly apply cosmetics, ineptly use various procedures, without having exact information on their real influence and dividing numerous delusions about it. All this not the best...
Section: Articles about health
Eyes – unique body on the structure thanks to which the person obtains about 80% of information on the world around: about a form...
Section: Articles about health
The state of health of the person in many respects depends on food. The organism will well function if during food it receive only useful substances, necessary vitamins and microelements. In this case there will be no problems with digestion, with лишн...
Section: Articles about health
Practically each person is familiar with the annoying, pulling, unscrewing pains caused by overcooling of muscles of a back. In certain cases inflammatory process is not limited to discomfort, being followed by emergence of hypostasis, consolidations, temperature increase. At the wrong treatment the acute miositis can lead to a chronic disease or aggravation of other pathologies of a back (vertebral hernia, osteochondrosis) therefore it is important to pay attention to symptoms of an illness in time and to start to...
Section: Articles about health