Dormikum
Application instruction:
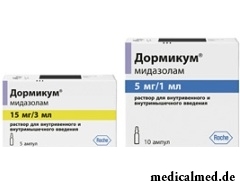 Dormikum – the sedative and somnolent drug used for premedication and an introduction anesthesia.
Dormikum – the sedative and somnolent drug used for premedication and an introduction anesthesia.
Form of release and structure
Dormikum release in the form of solution for intravenous and intramuscular administration: transparent, colourless or slightly yellowish (on 1 or 3 ml in colourless glass ampoules, on 5, 10 or 25 ampoules in a cardboard pack).
Is a part of 1 ml of injection solution:
- Active agent: midazolam – 5 mg;
- Auxiliary components: sodium chloride, Acidum hydrochloricum, sodium hydroxide, water for injections.
Indications to use
- Sedation with preservation of consciousness before, and also during the medical or diagnostic procedures made under local anesthesia or without it;
- Premedication before an introduction anesthesia;
- Long sedation in an intensive care;
- Introduction anesthesia at adults;
- The combined anesthesia (as a sedative component) at adults.
Contraindications
Absolute:
- Coma, shock, acute drunkenness with oppression of the vital functions;
- Acute respiratory and/or pulmonary insufficiency;
- Chronic obstructive pulmonary disease (at a heavy current);
- Closed-angle glaucoma;
- Period of childbirth;
- Hypersensitivity to drug components.
Relative (during Dormikum's use care is required):
- Myasthenia (myasthenia gravis);
- Respiratory and/or heart failure;
- Critical conditions;
- Functional disturbances of a liver and kidneys;
- Prematurity at children (because of danger of an apnoea);
- Children's age up to 6 months;
- Advanced age (of 60 years).
Data to estimate safety of use of Dormikum for pregnant women, are not enough. At this group of patients it is possible to appoint drug only in cases if there is no safer alternative. Dormikum's use in high doses in the third trimester of pregnancy or during the first period of childbirth can cause disturbances of a cordial rhythm in a fruit, hypotonia, suction disturbances, a hypothermia and moderate respiratory depression in the newborn. At children whose mothers at late stages of pregnancy for a long time received benzodiazepines, physical dependence with a certain risk of emergence of an abstinence syndrome during the post-natal period can be created.
Route of administration and dosage
Dormikum is among the strong sedatives demanding individual selection of a dose and slow introduction. For safe achievement of the necessary sedative action the titration of a dose corresponding to clinical need, age and a physical condition of the patient, and also the drug treatment received by it is insistently recommended.
At critical conditions, a high risk, at patients at children's age and at patients 60 years a dose are more senior it is necessary to choose carefully taking into account individual risk factors.
The effect of Dormikum's use develops approximately in 2 minutes after intravenous administration. The maximum action is reached within 5-10 minutes.
Sedation with consciousness preservation
Before holding diagnostic or surgical procedures for sedation with consciousness preservation the drug should be administered intravenously (to administer the drug struyno or quickly does not follow). Approach of sedation depends on a condition of the patient and the mode of dosing (size of a dose, rate of administering). In need cases the drug is administered repeatedly. It is necessary to be careful at patients with disturbances of respiratory function.
Dormikum adults need to enter intravenously slowly, with a speed about 1 mg / 30 seconds. An initial dose for patients 60 years (2-2,5 mg) are younger enter in 5-10 minutes prior to the procedure. According to indications the drug is administered repeatedly in a dose of 1 mg. The average total dose makes 3,5-7,5 mg.
To the patients who are in critical condition, patients with a high risk, and also to persons at the age of 60 years is also more senior an initial dose lower up to 0,5-1 mg and enter it in 5-10 minutes prior to the procedure. According to indications perhaps repeated introduction of the same dose. For achievement of effect usually happens rather total dose of 3,5 mg.
To children intravenous administration of Dormikum is carried out by slow titration before achievement of the necessary effect. The initial dose is entered for 2-3 minutes. Before introduction of the second dose it is recommended to wait for 2-5 minutes that will give the chance to estimate sedation. In need of its strengthening the dose should continue to be titrated small "steps". Use considerably of high doses, than to teenagers and children of advanced age can be required by babies and children up to 5 years.
Dormikum's use for sedation with preservation of consciousness at children up to 6 months is not recommended, except cases when the potential advantage is higher than possible risks, at the same time it is necessary to enter minimal effective doses.
The initial dose for children from 6 months to 5 years varies from 0,05 to 0,1 mg/kg. Its increase up to 0,6 mg/kg is possible (but no more than 6 mg, because of probable risk of emergence of hypoventilation and long sedation).
The initial dose for children of 6-12 years varies from 0,025 to 0,05 mg/kg. Its increase up to 0,4 mg/kg is possible (but no more than 10 mg, because of probable risk of emergence of hypoventilation and long sedation).
To children of 13-16 years usually appoint adult doses.
At intramuscular introduction to children of 1-16 years in 5-10 minutes prior to the procedure usually enter 0,05-0,15 mg/kg. As a rule, the total dose does not exceed 10 mg.
At body weight to 15 kg Dormikum's introduction with concentration more than 1 mg/ml are not recommended. Solutions with higher concentration need to be dissolved to concentration 1 mg/ml.
Premedication
For premedication Dormikum is entered intravenously or intramusculary (it is deep in a muscle in 20-60 minutes prior to an introduction anesthesia). Drug has sedative effect in the form of drowsiness and elimination of emotional pressure.
Identification of symptoms of overdose after administration of solution requires obligatory observation of a condition of the patient.
Dormikum it is possible to apply along with anticholinergic drugs.
The dosing mode is recommended to the following:
- Adults are younger than 60 years which belong to the class I and II on system of assessment of the physical status American Society of Anaesthesiologists: intravenously – 1-2 mg, if necessary repeat administration of drug; intramusculary – 0,07-0,1 mg/kg;
- Adult 60 years are also more senior, patients in critical condition or with a high risk: intravenously – 0,5 mg, in need cases increase a dose slow titration, before introduction of a repeated dose it is necessary to wait 2-3 minutes; intramusculary – 0,025-0,05 mg/kg (as a rule, 2-3 mg), at simultaneous use with drugs a dose it is necessary to reduce;
- Children of 1-15 years: intramusculary – 0,08-0,2 mg/kg (in 30-60 minutes prior to an introduction anesthesia deeply in a large muscle), at body weight to 15 kg are not recommended Dormikum's introduction with concentration more than 1 mg/ml.
Introduction anesthesia
At Dormikum's introduction for an introduction anesthesia before other anesthetics individual reaction of patients can be various. The dose needs to be titrated before achievement of desirable effect. At Dormikum's introduction before or along with other inhalation or intravenous means for an introduction anesthesia initial doses of each of drugs can be lowered considerably.
Desirable level of sedation is reached by step titration of a dose.
The induction dose of Dormikum needs to be entered intravenously slowly, fractionally. Introduction of each subsequent dose (no more than 5 mg) needs to be carried out within 20-30 seconds with breaks 2 minutes.
The dosing mode is recommended to the following:
- Adults up to 60 years: intravenously for 20-30 seconds in a dose of 0,2 mg/kg, waiting for 2 minutes for action assessment. In cases of lack of premedication it is possible to increase a dose to 0,3-0,35 mg/kg of body weight, it is entered intravenously for 20-30 seconds, waiting for 2 minutes for effect assessment. For completion of induction, if necessary, Dormikum enter in addition in the number of equal about 25% of an initial dose. As an alternative for completion of induction it is possible to apply liquid inhalation anesthetics. At immunity the induction dose is increased to 0,6 mg/kg, however in this case recovery of consciousness can be slowed down;
- Adult 60 years are also more senior, patients in critical condition or with a high risk: in the absence of premedication – 0,15-0,2 mg/kg, in the presence of premedication – for 0,05-0,15 mg/kg for 20-30 seconds intravenously, waiting for 2 minutes for effect assessment.
The combined anesthesia (as a sedative component)
- Adults up to 60 years: fractional intravenous administration of small doses (0,03-0,1 mg/kg) or continuous intravenous infusion in a dose of 0,03-0,1 mg/kg an hour (as a rule, along with analgetics). Doses and breaks between introductions are defined by individual reaction of the patient;
- Adult 60 years are also more senior, patients in critical condition or with a high risk: similarly, but using smaller doses.
Long sedation in an intensive care
The necessary sedation can be reached step titration of a dose then appoint either continuous infusion, or fractional jet introduction of Dormikum (on the basis of age and a condition of the patient, clinical requirement and at the same time entered medicines).
The adult the load dose of 0,03-0,3 mg/kg is entered intravenously fractionally, slowly. Each repeated dose of 1-2,5 mg is entered within 20-30 seconds with observance of two-minute breaks.
Against the background of a hypovolemia, vasoconstriction or hypothermias reduce a load dose or do not enter in general.
At combined use with strong analgetics (in particular, morphine, methadone, pethidine, fentanyl, alfentanil, buprenorphine, pentazocine and derivatives of each subgroup) they need to be entered before Dormikum's introduction.
The intravenous maintenance dose can vary from 0,03 to 0,2 mg/kg an hour. Against the background of a hypovolemia, vasoconstriction or hypothermias reduce a maintenance dose. It is recommended to estimate sedation degree regularly. When carrying out long sedation tolerance because of what the dose is increased can develop.
Including premature, and also to children with body weight to 15 kg administration of solution of Dormikum with concentration more than 1 mg/ml is not recommended to newborns.
To children up to 6 months solution is entered by continuous intravenous infusion.
The newborn with gestational age to 32 weeks Dormikum appoint in an initial dose 0,03 mg/kg an hour (0,0005 mg/kg a minute); of 32 weeks and to children up to 6 months – in a dose of 0,06 mg/kg an hour (0,001 mg/kg a minute).
The load dose is intravenously not entered, instead in the first several hours infusion is carried out slightly quicker that allows to reach therapeutic concentration of Dormikum in plasma. Careful control of a respiration rate and saturation by oxygen is necessary.
To children of 6 months which are on artificial ventilation of the lungs and also intubated, the load dose in a dose of 0,05-0,2 mg/kg is entered intravenously slowly not less, than for 2-3 minutes (it is impossible to enter intravenously quickly). After that pass to continuous intravenous infusion in a dose 0,06-0,12 mg/kg an hour (0,001-0,002 mg/kg a minute). For strengthening or maintenance of desirable effect increase or reduction in the rate of infusion or introduction of additional doses of drug is possible.
Against the background of disturbances of a hemodynamics, the usual load dose should be titrated small "steps", exercising control of hemodynamic indicators (lowering of arterial pressure). Such patients have a tendency to respiratory depression at Dormikum's use in this connection it is necessary to control carefully a respiration rate and saturation by oxygen.
Dosing in special cases
Including premature, and also to children with body weight less than 15 kg are not recommended to newborns administration of solutions of Dormikum with concentration more than 1 mg/ml. The solutions having higher concentration need to be dissolved to concentration 1 mg/ml.
Intravenous administration of midazolam is not recommended to children up to 6 months as they in particular are inclined to hypoventilation and obstruction of respiratory tracts, except for cases of carrying out sedation in chambers of an intensive care.
To patients of 60 years usually appoint lower doses, at the same time it is necessary to control indicators of the vital functions constantly.
The prepared solution should be used immediately. If necessary its storage for 24 hours at a temperature of 2-8 °C is possible.
Side effects
- Central and peripheral nervous system: an ataxy, long sedation, decrease in concentration of attention, an ecmnesia which duration depends on the entered dose (can arise at the end of the procedure, in certain cases proceeds longer), dizziness, a headache, postoperative drowsiness, uneasiness, retrograde amnesia, nonsense and drowsiness at an exit from an anesthesia, a sleep disorder, the atetoidny movements, the indistinct speech, a dysphonia, paresthesia; newborn and premature children have spasms;
- Immune system: acute anaphylaxis, reactions of generalized hypersensitivity (bronchospasm, cardiovascular, skin reactions);
- Cardiovascular system: in rare instances – the heavy cardiorespiratory undesirable phenomena (a cardiac standstill, a lowering of arterial pressure, bradycardia, a vazodilatation; the probability of development of these life-threatening reactions above at patients is more senior than 60 years and at patients with the accompanying respiratory and/or heart failure, in particular, if Dormikum is entered in a high dose or too quickly), a bigeminal pulse, tachycardia, vazovagalny crisis, premature reduction of ventricles, a rhythm of atrioventricular connection;
- Digestive tract: a lock, nausea, vomiting, hypersalivation, dryness and acid smack in a mouth, an eructation;
- Mental sphere: euphoria, confusion of consciousness, hallucination. There are messages on paradoxical reactions in the form of agitation, an involuntary physical activity (including toniko-clonic spasms and a muscular tremor), hyperactivities, hostile mood, anger and aggression, excitement paroxysms, in particular at children and patients at senile age. Even therapeutic doses of Dormikum can lead use to formation of physical dependence (the risk of its development increases at increase in a dose of drug and duration of its use, and also at patients with alcoholism and/or having drug addiction in the anamnesis). Dormikum's cancellation, in particular sharp, after its long intravenous use, can cause cancellation symptoms, including spasms;
- Skin and hypodermic fatty tissue: urticaria, skin rash, itch;
- Respiratory organs: in rare instances – the heavy cardiorespiratory undesirable phenomena (oppression, an apnoea, development диспноэ, an apnoea, a laryngospasm; the risk of their development is higher at adults of 60 years and at patients with the accompanying respiratory and/or heart failure, in particular, if Dormikum is entered in a high dose or too quickly), shallow breathing, a hiccups, a bronchospasm, goose breathing, obstruction of respiratory tracts, a hyperventilation, a tachypnea;
- Sense bodys: a congestion in ears, deterioration and disturbance of visual acuity, a nystagmus, doubling in eyes, periodic twitchings a century, miotic pupils, disturbance of a refraction, a preunconscious state, balance loss;
- Local and general reactions: pains and an erythema in the place of an injection, fibrinferments, thrombophlebitis, hypersensitivity.
At elderly patients after Dormikum's use the probability of falling and changes increases.
Special instructions
At Dormikum's use in the conditions of a hospital of one day of the patient it is possible to write out only after survey of the anesthesiologist.
When carrying out premedication after Dormikum's introduction it is necessary to watch a condition of the patient as individual sensitivity to active agent can differ surely.
Drug can lead to development of an ecmnesia. Long amnesia can constitute danger to patients who are going to be written out after the diagnostic or surgical procedure.
As sharp cancellation of Dormikum can be followed by abstinence signs, the dose of drug is recommended to be reduced gradually.
It is necessary to avoid use of midazolam for the patients with alcoholism and also having drug addiction in the anamnesis. Patients need to observe extra care at administration of drug with myasthenia gravis.
Before complete cessation of effect of Dormikum to manage vehicles or to work with machines or mechanisms does not follow. Resuming of such activity is possible after permission of the attending physician.
Medicinal interaction
It is necessary to be careful at co-administration of Dormikum with rifampicin, ketokonazoly, flukonazoly, itrakonazoly, pozakonazoly, erythromycin, klaritromitsiny, sakvinaviry, Cimetidinum, diltiazem, atorvastatiny, fluvoksaminy, nefazodony, aprepitanty, hlorzoksazony, bikalutamidy, a goldenseal Canadian (Hydrastis Canadensis), carbamazepine/Phenytoinum, efavirenzy, extract of a root of a purple cone-flower purple, a St. John's Wort ordinary, valproic acid.
Joint therapy with other hypnotic drugs and sedatives, including alcohol, can cause strengthening of somnolent and sedative effects; with the drugs oppressing the central nervous system – strengthening of sedation and influence on indicators of a hemodynamics and respiratory system; the drugs activating activity of a brain, improving memory, attention – reduction of somnolent effect.
Carrying out spinal anesthesia can lead to strengthening of sedation of midazolam.
It is not necessary to part Dormikum 6% with dextran solution with an average molecular weight of 50 000-70 000 Da in Dextrosum. It is impossible to mix midazolam with alkaline solutions.
Terms and storage conditions
To store in protected from light, the place, unavailable to children, at a temperature up to 30 °C.
Period of validity – 5 years.
In operating time our brain spends the amount of energy equal to the 10 Watts bulb. So the image of a bulb over the head at the time of emergence of an interesting thought is not so far from the truth.

Dark circles (bruises) under eyes – a shortcoming with most of which often fight against the help of cosmetics (proofreaders, salons...
Section: Articles about health
Cold, puffiness of a nose, itch, the watering eyes - characteristic symptoms of the allergic rhinitis resulting from hit of allergens (pollen, house dust, hair of animals, etc.) on a mucous membrane of a nose. Unpleasant feelings often deliver беспоко...
Section: Articles about health
We present to yours the TOP of the medicamentous means exerting the stimulating impact on a potentiality, i.e. on ability of the man to commission of sexual intercourse. At once it is necessary to tell that not always disturbances of erectile function can be eliminated with reception of this or that drug. The reasons of decrease in a potentiality there can be a set, from banal overfatigue before tumoral process in a small basin therefore if the man faces similar problems too often, it should turn...
Section: Articles about health
They say that to ensure health and longevity of people it is obliged. Really, at competent approach to these questions, we will pass...
Section: Articles about health
Herpes simplex of the first type (the infectious disease which is shown periodic bubble rashes on lips is called) – one of the most widespread illnesses. Statistically, only 5% of inhabitants of our planet are unreceptive to its activator, and...
Section: Articles about health
Contrary to popular belief, the multiple sclerosis (MS) is not connected neither with sclerous changes of walls of vessels, nor with age forgetfulness and problems with concentration of attention. This disease has the autoimmune nature. Pathological process is expressed in degradation of nervous tissue and destruction of its enveloping layer - a myelin. Multiple damages of the central nervous system which are shown by decrease in sight, bystry fatigue, on become result of development of an illness...
Section: Articles about health
Epilepsy is one of widespread neurologic diseases. To parents, whose children suffer from this illness, it is necessary...
Section: Articles about health
Zone hypostases under eyes - very widespread problem giving to people is a lot of inconvenience. Hypodermic fabric in these parts has very loose structure and almost does not contain collagenic fibers. Besides, the skin covering подглазья constantly is exposed...
Section: Articles about health
There comes the season of issues. Many Russians already dream of outdoor recreation, trips, beautiful seaside beaches. At this time there is no wish to think of problems with health and other unpleasant things, however there are subjects which require attention. In the summer repeatedly the risk increases to ache with some very dangerous illnesses, we also will talk about them today....
Section: Articles about health
For many spouses the question of planning of a family is one of the main. The choice problem effect at the same time comes out on top...
Section: Articles about health
Women quite often suffer from complexes concerning the sizes of the bust. Strangely enough, not too modest, and excessively curvy shapes become the reason of sincere discomfort sometimes. Except psychological problems, a big bust sometimes with...
Section: Articles about health
The modern person not always manages to find housing in the environmentally friendly region and such work which would not do harm to health. With food stuffs at first sight the situation is much better: shops are overflowed with goods which are positioned by producers as very useful and absolutely safe. Many Russians are absolutely sure that the choice of products with marking "bio", "эко" or "organik" guarantees them and members of their families an optimal variant of food. To a sozhala...
Section: Articles about health
Residents of big cities quite often have a disease which is known as the syndrome of chronic fatigue (SCF) today. This illness...
Section: Articles about health
Smoking not only exerts a negative impact on the state of health of the consumer of tobacco products, but is an air polluter the substances potentially dangerous to people around. In recent years significantly the number of people, стремящ increased...
Section: Articles about health
High temperature - a frequent symptom of such widespread diseases as a SARS, quinsy, pneumonia, etc. To reduce heat, having facilitated a condition of the patient, doctors recommend to accept antipyretics, however their use is not always possible. Too frequent use of these drugs can lead to allergic reactions, and also overdose, causing poisoning. It happens also that there are no antipyretics simply in the house. In these situations it is pertinent to use it...
Section: Articles about health
Hemorrhoids – extremely widespread disease. Periodically arising inflammations and bleeding of hemorrhoidal nodes пр...
Section: Articles about health
Tuberculosis – a serious infectious disease which development is caused by mycobacteria (Koch's bacilli). The illness is known from an extreme antiquity. Long time fight against it was considered as ineffective. Quite often the disease affected the whole families, and mortality from it was very much...
Section: Articles about health
The sudden heat on all body which is followed by perspiration and a cardiopalmus – the phenomenon familiar to many people. Most often such states called by "inflows" result from nervous or physical overworks and disappear right after rest. However in certain cases similar reaction of an organism can speak about diseases which need treatment. What? About it below....
Section: Articles about health
Cold is such painful that each sigh becomes a victory, heat "knocks" down, and the ache in joints forces to think only about...
Section: Articles about health
Vitamin complexes belong to the most popular drugs, probably, in our country there is no person who was not hearing about advantage of vitamins and never their accepting. The more vitamins, the better, we consider and as it appeared, cruelly we are mistaken. So l...
Section: Articles about health
Ayurveda - the most ancient tselitelsky practice which came to us from India. It represents the doctrine about maintenance of physical, psychological and moral health of the person by means of the complex of procedures including a diet, cleaning of an organism, breathing exercises, massage, and in case of a disease - and medicinal therapy. The healers practicing Ayurveda assign very important part to spices, and at the heart of Ayurvedic drugs, as a rule, there are they. It is considered that spices not of t...
Section: Articles about health
Extracorporal fertilization – one of the most modern methods of controlling with infertility. So far it already helped znach...
Section: Articles about health
History of use of an anesthesia during operations contains more than 160 years. Annually in the world hundreds of thousands of surgical interventions during which to patients the substances immersing them in a dream and saving from pain are entered are carried out. Using an anesthesia to these...
Section: Articles about health
Health and attractiveness - eternal values, pursuing which people often use the most unusual ingredients and technicians. Let's consider 11 most exotic and sometimes not most pleasant Spa procedures to which the person in a pursuit of beauty and youth agrees....
Section: Articles about health
Stroke (acute disorder of cerebral circulation) – one of the most widespread neurologic diseases. Annually in the world...
Section: Articles about health
The list of stereotypes of which, apparently, all know strongly includes following: British surely eat porridge for breakfast. Perhaps, not all modern residents of Britain arrive quite so, but for those from them which continue to follow this t...
Section: Articles about health
About 10-15 years ago existence of the computer in the apartment of the Russian was considered as a rarity and office rooms were only at the first stage of equipment by these useful devices. Today practically in each house there is a computer (and often not one), and a regular user is already every our second compatriot. Convenience and efficiency of personal computers are undoubted, but the people working with them daily have to know also about health hazard which they can predstavlit...
Section: Articles about health