Karboplatin-Ebev
Application instruction:
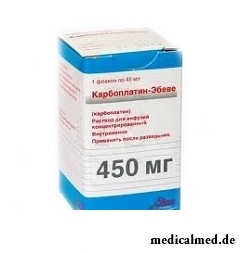 Karboplatin-Ebev – drug with antineoplastic action.
Karboplatin-Ebev – drug with antineoplastic action.
Form of release and structure
Karboplatin-Ebev release in the form of a concentrate for preparation of solution for infusions: transparent, colourless or almost colourless (in brown glass bottles on 5, 15, 45, 60 or 100 ml, on 1 bottle in a cardboard pack).
For preparation of solution for infusions is a part of 1 ml of a concentrate:
- Active agent: карбоплатин – 10 mg;
- Auxiliary components: sodium hydroxide, anhydrous dihydrosodium phosphate, water for injections.
Indications to use
- Germinogenny tumors at women and at men;
- Ovarian cancer;
- Cancer of a neck of uterus;
- Lung cancer;
- Bladder Schmincke's tumor;
- Malignant tumors of a neck and head.
Contraindications
- The expressed functional disturbances of kidneys (at clearance of creatinine of ≤15 ml a minute);
- Plentiful bleedings;
- The expressed miyelosupressiya;
- Pregnancy and period of a lactation;
- Hypersensitivity to drug components, and also to other platiniferous connections.
Route of administration and dosage
Karboplatin-Ebev it is possible to apply as monotherapy or along with other antineoplastic drugs. The doctor sets the dosing mode individually. The drug is administered intravenously in the following dose modes:
- 300-400 mg/m ² intravenously kapelno for 15-60 minutes or in the form of 24-hour infusion;
- 100 mg/m ² intravenously kapelno for 15-60 minutes daily within 5 days.
Administration of drug it is necessary to repeat with an interval not less than 4 weeks at indicators of neutrophils not less than 2000 cells/mm ³ blood and thrombocytes not less than 100000 cells/mm ³ blood. To or after drug use administration of liquid and an artificial diuresis is not required.
The therapeutic dose of Karboplatin-Ebev depending on function of kidneys or a condition of marrow can korrigirovatsya as follows:
- Patients with risk factors (for example, after performing myelosuppressive therapy or at the low functional status): the initial dose should be lowered by 20-25%;
- Patients with functional disturbances of kidneys (at clearance of creatinine it is less than 60 ml in a minute): because of the increased risk of development of a heavy miyelosupressiya the dose of drug needs to be lowered (at clearance of creatinine of 41-59 ml a minute – to 250 mg/m ², at clearance of creatinine of 16-40 ml a minute – to 200 mg/m ²);
- Patients with symptoms of heavy or moderate hematologic toxicity (at quantity of thrombocytes and neutrophils less than 50000 and 500/mm ³ respectively): decrease in a dose by 25% (can be required at monotherapy and the combined scheme of treatment);
- Patients are more senior than 65 years: adjustment initial and the subsequent doses can be required.
These recommendations about the mode of dosing belong to an initial course of treatment. Further doses need to be adjusted depending on Karboplatin-Ebev's portability and development of a miyelosupressiya.
The initial dose of drug in mg can be determined by Kalvert's formula which describes dependence of values of speed of glomerular filtering (SGF in ml/min.) and desirable concentration of Karboplatin-Ebev from time (AUC in mg/ml × mines):
General Dose (mg) = AUC x (SGF + 25)
Desirable AUC value:
- 5-7 мг/мл×мин: when performing monotherapy at earlier not treated patients;
- 4-6 мг/мл×мин: when performing monotherapy at earlier treated patients or when performing the combined treatment (with cyclophosphamide) at earlier not treated patients.
Before introduction Karboplatin-Ebev it is necessary to dilute to concentration 0,5 mg/ml of 0,9% with solution of sodium of chloride or 5% dextrose solution.
Weak solution of drug keeps stability for 8 hours at a temperature of 25 °C and 24 watch at its storage in the refrigerator at 4 °C.
Side effects
During Karboplatin-Ebev's use development of the following side effects is possible:
- Bodies of a hemopoiesis: the major toxic factor limiting a dose of a karboplatin is suppression of function of a marrowy hemopoiesis. Miyelosupressiya has dozozavisimy character. As a rule, the lowest level of thrombocytes and granulocytes/leukocytes is reached in 2-3 weeks from the moment of the beginning of use of Karboplatin-Ebev, at the same time thrombocytopenia meets more often. Usually recovery to the level necessary for introduction of the following dose of drug, takes not less than four weeks. At rather large number of patients symptoms of anemia can be also shown (hemoglobin level less than 11 g/dl). Its intensity depends on a total dose of drug. In certain cases performing transfusion therapy, especially at patients can be required, it is long using drug (for example, it is more than 6 cycles). Also there is a probability of development of such clinical complications as: infectious diseases, fever, bleeding, septic shock / sepsis;
- Digestive tract: in the first 6-12 hours after Karboplatin-Ebev's introduction there is a probability of developing of vomiting and/or nausea (from easy to moderated) which can proceed till 24 o'clock and more. The risk of emetic effect can be reduced at use of antiemetics, continuous intravenous infusion of a karboplatin within 24 hours or fractional introduction of the appointed dose within 5 days in a row. In certain cases can also arise: diarrhea, inflammation of a mucous membrane of a mouth, abdominal pains, locks;
- Central and peripheral nervous system: there is a risk of development of the peripheral neuropathies which are shown, mainly, as paresthesias and decrease in deep tendon jerks (most possibly for patients 65 years at the long or previous treatment are more senior Cisplatinum). Emergence of signs of dysfunction of the central nervous system is also possible. Prolonged use of drug can lead to a cumulative neurotoxicity;
- Acoustic organ: ototoxicity (sonitus and deterioration in hearing);
- Organ of sight: there is a risk of temporary deterioration or total loss of sight (loss of ability to see light is possible and to distinguish colors), and also other disturbances of visual function. As a rule, the complete recovery and/or improvement of sight occurs within several weeks after the therapy termination. At patients with functional disturbances of kidneys the cortical blindness can develop;
- Kidneys: development of easy and temporary increase in concentration of urea and creatinine in blood serum is possible. Acute damages of kidneys are observed in rare instances. The risk of emergence of nephrotoxicity against the background of use of a karboplatin (decrease in clearance of creatinine) increases at increase in a dose of drug, and also at patients who underwent treatment by Cisplatinum earlier;
- Liver: perhaps easy and, as a rule, short-term increase in concentration of aspartate aminotransferase, an alkaline phosphatase and bilirubin in blood serum. Considerable abnormal liver functions can arise at the patients receiving high doses of drug with autologichesky transplantation of marrow;
- Electrolytic balance: the hypocalcemia, a hypopotassemia, a hypomagnesiemia and/or a hyponatremia are possible;
- Allergic reactions: fever, erythematic rash, itch, bronchospasm, small tortoiseshell, anaphylactic reactions, arterial hypotension. These reactions can be observed already in a few minutes after administration of drug. In rare instances exfoliative dermatitis can develop;
- Others: an alopecia, taste changes, an adynamy, grippopodobny symptoms (fever, increase in temperature), a gemolitiko-uraemic syndrome, an arthralgia / миалгия, cerebrovascular disturbances, heart failure and allergic reactions directly in a drug injection site.
Special instructions
The doctor having experience of use of cytotoxic drugs has to carry out Karboplatin-Ebev's introduction. During therapy it is necessary to exercise constant control of development of possible toxic effects, especially at use of high doses.
During Karboplatin-Ebev's use men and women need to use reliable ways of contraception.
To use to preparation and administration of solution syringes, needles, infusional systems and catheters with the content of aluminum does not follow as it can react with active agent, leading to loss of its activity or formation of a deposit.
Once a week it is necessary to control uniform elements of peripheral blood and indicators of function of a liver and kidneys (the clearance of creatinine belongs to the most sensitive indicators).
Also it is recommended to perform periodically neurologic inspections, especially at patients 65 years and at the patients undergoing earlier therapy by Cisplatinum are more senior.
As карбоплатин can lead to development of cumulative ototoksichesky effects, to patients prior to the beginning of and during treatment audio is recommended to pass graphic researches. At clinically significant dysfunctions of hearing the termination of therapy or change of a dose of drug can be required.
During therapy it is necessary to observe all usual instructions accepted for use of cytotoxic medicines.
Medicinal interaction
At simultaneous use of Karboplatin-Ebev with some medicines there can be undesirable effects:
- Other myelosuppressive drugs or radiation therapy: increase in risk of development of hematologic toxicity;
- Aminoglycosides and other nephrotoxic drugs: increase in risk of emergence of ototoksichesky and/or nephrotoxic effects.
Terms and storage conditions
To store in protected from light, the place, dry, unavailable to children, at a temperature up to 25 °C.
Period of validity – 3 years.
At regular visit of a sunbed the chance to develop a carcinoma cutaneum increases by 60%.

Smack in a mouth can arise in the natural way – as a result of lack of morning hygiene or reception of the corresponding food. Odn...
Section: Articles about health
Phobia – the persuasive fear of a certain contents shown in a specific situation against the will of the person. Concepts of a phobia and fear are similar, however if the fear is natural protective function of mentality, then the phobia is its deviation. So the person can an ispytyva...
Section: Articles about health
Producers of milk mixes for children assure: mixes are ideally balanced and adapted for needs of babies. If mother should raise artificially the kid owing to serious problems with health, to do nothing – it is necessary to feed with substitutes of milk. However pediatricians note that not seldom women without good reasons refuse feeding of the child a breast and pass to milk mixes. Common causes of such decision – the aspiration to leave quicker...
Section: Articles about health
Such trouble as the milkwoman's attack, at least once in life happened almost to each woman. Prevalence забол...
Section: Articles about health
The kid who was recently born is surrounded with love of adult family members and their cares without which the baby cannot exist. Some parents consider that gentle attachment and caress are quite enough that the child correctly developed and was happy...
Section: Articles about health
The body of the person almost for 60% consists of water. It is so important for normal functioning of an organism that loss of only one and a half percent of liquid already leads to the most unpleasant effects. The problems connected with deficit of water can overtake also the healthiest person if he, for example, spends several hours under the scorching sun, without having taken with themselves drink, but is very simple to correct health in this case. It is much more difficult to minimize effects of other reasons about...
Section: Articles about health
High temperature - a frequent symptom of such widespread diseases as a SARS, quinsy, pneumonia, etc. To reduce heat, облег...
Section: Articles about health
Sugar - the digestible refined product which is not of special value for an organism of the modern person. The use of sugar in food is based rather on the psychological dependence caused by desire to indulge itself with something tasty, and in дальнейш...
Section: Articles about health
Scientists always aimed to offer fundamental explanations for medical problems. Their theories formed the basis of modern methods of treatment of the hardest pathologies and helped to save a set of lives. However stories are known also such theoretical constructions, following to which brought to mankind of a trouble and torture, ruined destinies and health of many people....
Section: Articles about health
Life expectancy in various regions of Earth is not identical. Exert impact on it social stability, economic бл...
Section: Articles about health
Striya (extension) are the defects of skin having an appearance of direct or wavy strips from 1 to 10 cm long and 1-5 mm wide. In most cases at women of a striya are located on a stomach, hips, a breast or buttocks. At athletes they can appear on shoulders and внутренн...
Section: Articles about health
There is a lot of fans of beer in our country. Statistically, on each average Russian (including women and children) in a year about 60 liters of this drink are consumed. It is not a lot of, as in the Czech Republic or Germany, but figure all the same impressive. There is nothing to rejoice here: despite assurances of producers that beer is absolutely harmless, effects of its active consumption cannot be considered positive in any way. Here only part of that negative impact, which popular нап...
Section: Articles about health
For the last decades the diabetes mellitus of the second type became really world problem. Number of cases annually cart...
Section: Articles about health
Herpes simplex of the first type (the infectious disease which is shown periodic bubble rashes on lips is called) – one of the most widespread illnesses. Statistically, only 5% of inhabitants of our planet are unreceptive to its activator, and...
Section: Articles about health
Cellulitis - very widespread cosmetic shortcoming which arises approximately at 80% of women sooner or later. Emergence it is connected with change of structure of a hypodermic fatty layer. At the same time on the surface of skin at first there are roughnesses (cambers and cavities), and then small consolidations, the so-called effect of an orange-peel is shown. Changes in a condition of hypodermic cellulose are a consequence of a hormonal imbalance in an organism....
Section: Articles about health
The sclera and mucous membrane of an eye are intensively supplied with blood vessels which problem - to sate nervous tissues of body to a pitata...
Section: Articles about health
For the time being the perspective of heart diseases seems to most of people remote and foggy. But sooner or later practically each adult faces extremely unpleasant feelings: sudden stethalgia. To be consoled at this time in a thought of t...
Section: Articles about health
The saying "the rich do not know how the other half lives" is known to all. In a broad sense it is that we can not always understand the person whose features of a state are unknown to us. If with physiological characters of diseases the situation is more or less clearly (having noticed them, we realize that to the person nezdorovitsya), then with symptoms of the illnesses affecting the mental sphere everything is much more difficult. Not absolutely usual behavior is quite often perceived surrounding as a ridiculous eccentricity, or that much ху...
Section: Articles about health
Each person has easy indispositions which he transfers "standing", trying not to ask for medical care. Argu...
Section: Articles about health
The climax, or menopause is the normal process of the termination of genital function of the woman which is followed by serious hormonal changes in an organism. Usually the menopause begins at the age of 50-55 years, but characteristics of this process are very individual. T...
Section: Articles about health
Partial and the more so full loss of hearing significantly reduces quality of life. Difficulties with communication lead to loneliness and isolation. The person who badly hears experiences difficulties with social and professional implementation, quite often has problems in private life....
Section: Articles about health
Wood louse – the ordinary-looking unpretentious plant extended in all territory of our country. It quickly expands, and sometimes for...
Section: Articles about health
For residents of the countries of Southeast Asia various algas are an obligatory component of a daily diet. Their popularity is connected not only with high tastes, but also with numerous curative properties. Russians are a little familiar with...
Section: Articles about health
Iodine - one of thirty most important microelements in our organism. The main role of iodine consists in synthesis of thyroid hormones of a thyroid gland - the substances which are responsible for the majority of exchange processes of an organism. It is known that thyroid hormones consist of iodine more than for 65%. The lack of iodine leads to decrease in production of hormones and, as a result, development of a hypothyroidism. The long condition of deficit can become a source of problems of the cardiovascular, bone, digestive SI...
Section: Articles about health
Frosty air, fresh wind and easy snowball at most of Russians are associated with cheerfulness, health and cheerful entertainments, on to...
Section: Articles about health
Vitamin complexes belong to the most popular drugs, probably, in our country there is no person who was not hearing about advantage of vitamins and never their accepting. The more vitamins, the better, we consider and as it appeared, cruelly we are mistaken. So l...
Section: Articles about health
Food with the increased content of sugar is attractive to most of people - it is scientifically confirmed fact. Business here not in intemperance or dissoluteness: the sweet food is associated since childhood with feeling of rest and safety which tests the kid when it absorbs maternal milk. Besides, getting into a human body, sugar strengthens production of "happiness hormones" which all of us so need. And still life of sweet teeth seldom happens cloudless: their too big loss...
Section: Articles about health