





Sodium nitrate
Sodium nitrate is known also under the name sodium or Chilean saltpeter. It has a chemical formula NaNO3 and in the cleared look represents white, grayish or slightly yellowish crystals of powder. Externally and on taste sodium saltpeter reminds usual table salt and is well dissolved in water. In the nature it is possible to meet this connection in the natural form – such natural mineral is spread to territories of Chile.
Sodium nitrate: terrible or nice
In most cases receiving sodium nitrate is based on synthesis of crystal substance from nitrogen of air or ammonia. Other technologies of receiving sodium nitrate represent various decomposition reactions or interactions of substances. For example, receiving sodium nitrate lixiviation from natural deposits hot water, crystallization, absorption of nitrogen oxides by solution of soda, decomposition of ammonium or calcic nitrate and the subsequent exchange with a carbonate, sulfate or sodium chloride is possible.
Sodium saltpeter is widely used in industrial production at production of corrosion-proof pipes, flew down, production of coolants and rocket fuel, and also is successfully applied in agriculture, medicine and the food industry. The medicines containing sodium nitrate are appointed as the bronchial spasmolytics, purgatives, means eliminating intestinal spasms and antidotes of cyanides.
By production of food stuffs, is more often than some sausages and cheeses, sodium saltpeter acts as the dye and preservative interfering distribution of the causative agent of a fatal disease – botulism; as nutritional supplement it is designated as E251. At the same time substance in high doses is extremely toxic for mammals, and when heating – heat treatment of products - has the proved cancerogenic activity.
For the person the highest daily dose of sodium nitrate makes 3,7 mg of substance on 1 kg of body weight, at the same time not the sodium, but nitric component is considered. So, at the body weight of 70 kg of 259 mg of E251 on the nitric NO3 group there will correspond about 350 mg, that is to exceed admissible norm.
In a human body sodium nitrate solution as a result of metabolism is capable to cause air hunger of bodies and fabrics, and in high doses of a message to a serious poisoning, a fluid lungs, an acute heart failure and even death. At interaction with proteins in the course of digestion such solution forms carcinogenic substances.
Symptoms of poisoning with the Chilean saltpeter are abdominal pains, a posineniye of integuments, especially in the field of a nasolabial triangle and nails, a headache, spasms, the complicated breath, nausea, vomiting, diarrhea.
Sodium nitrate solution: the dose solves everything
Modern reality such is that the hope for life without dyes and preservatives can be considered illusion. Control of the consumed products and dosages of similar substances is vital today. So, the sodium nitrate solution containing 8-15 mg/l of dry matter is deadly to the person. Totally ammonium nitrates, potassium, calcium and sodium, should not exceed:
- 45 mg/l in drinking water;
- 130 mg/kg in soil;
- 60 mg/kg in water-melons, pears and apples;
- 400 mg/kg in vegetable marrows;
- 500 mg/kg in a late white cabbage;
- 250 mg/kg in potatoes;
- 80 mg/kg in onions;
- 250 mg/kg in late carrots;
- 1400mg/kg in beet.
When processing a harvest it is necessary to avoid steam inhalation of any organic nitrates, their hit on skin and in a digestive tract.
The stomach of the person not bad copes with foreign objects and without medical intervention. It is known that the gastric juice is capable to dissolve even coins.

The concept "gluten" (differently, a gluten) combines group of the proteins which are a part of rye, barley and wheat. For most of people упот...
Section: Articles about health
Some people consider what for medicine of the 21st century of secrets in the field of health of the person almost does not exist. It absolutely not so. The more answers scientists receive, the more the most difficult questions are raised for them by life. Besides, there are diseases, not объясн in any way...
Section: Articles about health
Practice of hypnotic impact on consciousness of the person contains about two millennia. During this time scientists managed to learn a lot of things about a phenomenon of hypnosis and learned to facilitate a condition of the patients having heavy illnesses with its help....
Section: Articles about health
Statistically, can only one of ten of our compatriots brag of a decent condition of an oral cavity. On среднестатистич...
Section: Articles about health
What woman does not dream of a beautiful and thick hair? While physicians developed difficult schemes on hair transplant, in the industry of hairdresser's art a few years ago there was a sensation – methods of hair extension appeared. It would seem, dreams came true...
Section: Articles about health
Urogenital candidiasis (milkwoman) – a fungal infection which annoys unpleasant feelings in the field of generative organs, being followed by white curdled allocations, an itch, discomfort during an urination, pain. She is called by Candida fungus – the opportunistic organism living on mucous membranes of an organism....
Section: Articles about health
Wood louse – the ordinary-looking unpretentious plant extended in all territory of our country. It quickly expands, and sometimes for...
Section: Articles about health
One of the major chemical processes happening in a human body are oxidation reactions. They go with participation of fats and carbohydrates which we receive from food, and the oxygen getting to us from air. A main goal of such reactions is it is received...
Section: Articles about health
The phenomenon of improvement of a condition of the patients at administration of drugs who are not containing active agents, so-called effect of placebo is known long ago. At the end of the 18th century the American doctor Perkins began to treat people the "miracle" sticks made of alloy of steel and brass. Was for several minutes to press such subject enough to a sore point that it became much easier for the patient. Having suspected Perkins of charlatanism, his colleagues tried to repeat "miracle" by means of sticks, steles...
Section: Articles about health
The depression not without reason is considered one their main troubles of our century: for scientific and technical progress, acceleration of rate of life and a surplus...
Section: Articles about health
The pancreas performs two functions in a human body: release of enzymes without which digestion of carbohydrates, fats and proteins, and a producing hormones is impossible. The most important of them - insulin, is the main participant of carbohydrate metabolism, a normal...
Section: Articles about health
Not everyone can brag of the shining Hollywood smile. Even the person who is regularly visiting the stomatologist and watching of oral cavities over health periodically has problems: enamel of teeth darkens under the influence of some products, on it the deposits giving to teeth a grayish or yellowish shade collect....
Section: Articles about health
No, probably, the person who would not have cold. Cold, cough, a headache – these symptoms are known to everyone. Peak to Prost...
Section: Articles about health
Eyes – unique body on the structure thanks to which the person obtains about 80% of information on the world around: about a form, color, size, the movement, and also many other parameters of objects or phenomena. But whether much we know about the most valuable body...
Section: Articles about health
Many of us, probably, noticed more than once that from intellectual loadings at some point the brain as though "overheats" and "assimilation" of information is strongly slowed down. Especially this problem urgent for persons of age becomes more senior than fifty years. "Already badly I think", "the head will burst now", "memory as if is disconnected" - here that wants to be told at the time of information overload....
Section: Articles about health
Contrary to popular belief, the multiple sclerosis (MS) is not connected neither with sclerous changes of walls of vessels, nor about age...
Section: Articles about health
The thought that the mass of their body is too big at least once in life visits from 80 to 95% of women. Many women are so obsessed with this idea that constantly try all new and new ways of weight reduction. Considerable part of these method...
Section: Articles about health
Radiological methods of a research are applied in medicine more than hundred years, and thanks to them millions of lives were saved. In many cases without X-ray it is impossible to make exact idea of a condition of bodies and fabrics, it is correct to make the diagnosis. Nevertheless, many myths about researches such continue to exist. Let's consider the most widespread of them....
Section: Articles about health
The main role in development of a peptic ulcer of a stomach and duodenum the bacterium Helikobakter plays pilor. Activity and Wuxi...
Section: Articles about health
The hysteromyoma is diagnosed more than at a third of women 35 years are more senior. This high-quality new growth which at early stages successfully resolves by means of medicines. It is necessary to resort to an operative measure only in those a case...
Section: Articles about health
Dogrose – one of the most widespread adornment and medicinal plants growing practically in all territory of our country. To most of Russians it is a beautiful bush it is known, first of all, as a source of fruits, extremely vitamin-rich. However curative properties of a dogrose are not limited to it at all. About how still it is possible to use a plant in the medical purposes, we will tell today....
Section: Articles about health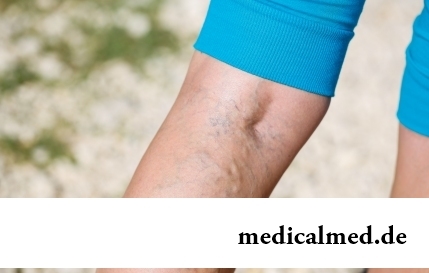
The varicosity has familiarly many, statistically, this disease more than a half of all adult population. As...
Section: Articles about health
Mushrooms - the surprising inhabitants of our planet having a set of wonderful qualities. Thanks to one of them, a mold mushroom of Penicillium notatum, the first natural antibiotic - penicillin was received nearly 80 years ago. The mankind is obliged to this opening миллио...
Section: Articles about health
Dietary supplements (dietary supplements) for the last decades were so thoroughly included into our life that, apparently, it is already impossible to find the person who at least once did not try them. At the same time, most of our compatriots have a vague idea of what dietary supplements as they affect a human body consist of and what differ from the real medicines in. Let's try to understand these questions, and at the same time and to understand, such additives are how necessary for us....
Section: Articles about health
Smack in a mouth can arise in the natural way – as a result of lack of morning hygiene or reception of the corresponding food. Odn...
Section: Articles about health
Condition of lips (their morbidity, outward) – one of indicators of health of the person. The peeling, dryness, pallor, and also cracks in corners of a mouth can be not only the cosmetic shortcoming which arose owing to physical damages and weather having sent away...
Section: Articles about health
Scientists always aimed to offer fundamental explanations for medical problems. Their theories formed the basis of modern methods of treatment of the hardest pathologies and helped to save a set of lives. However stories are known also such theoretical constructions, following to which brought to mankind of a trouble and torture, ruined destinies and health of many people....
Section: Articles about health
