





Advagraf
Application instruction:
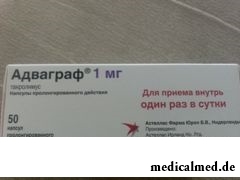 Advagraf – highly active immunodepressive medicine; inhibitor of a kaltsinevrin.
Advagraf – highly active immunodepressive medicine; inhibitor of a kaltsinevrin.
Form of release and structure
Dosage form of drug – the capsule of the prolonged action: gelatinous firm, filled with white powder (on 10 pieces in blisters from PVC / aluminum foil, in the aluminum soldered package on 5 blisters, 1 package in a cardboard pack):
- 0,5 mg: the size No. 5, a pale yellow cover with a red text of "0,5 mg", the orange case with figures "647" and a logo of the company;
- 1 mg: the size No. 4, a white cover with a red text "1mg", the orange case with figures "677" and a logo of the company;
- 3 mg: the size No. 1, an orange cover with a red text of "3 mg", the orange case with figures "637" and a logo of the company;
- 5 mg: the size No. 0, a grayish-red cover with a red text of "5 mg", the orange case with figures "687" and a logo of the company.
1 capsule of the prolonged action contains:
- Active ingredient: такролимус (in the form of a takrolimus of monohydrate) – 0,5 mg, 1 mg, 3 mg or 5 mg;
- Auxiliary components: gipromelloza, lactoses monohydrate, ethyl cellulose, magnesium stearate;
- Capsule cover: iron dyes (oxide yellow and oxide red (Е 172)), gelatin, titanium dioxide (Е 171), sodium lauryl sulfate;
- Ink for a text on the capsule (Opacode S-1-15083): glaze of pharmaceutical 45% (shellac solution in ethanol), a hypro rod, симетикон, lecithin (soy), dye ferrous oxide red.
Indications to use
Advagraf is shown to use for adult patients for the prevention of rejection of allotransplant of a kidney or a liver and for therapy of rejection of allotransplant, resistant to the standard modes of immunosuppressive therapy.
Contraindications
- Lactose intolerance, deficit of lactase, glyukozo-galaktozny malabsorption;
- Children's and teenage age up to 18 years (clinical experience of use is limited);
- The increased individual sensitivity to a takrolimus, other macroleads, auxiliary components of drug.
As safety of use of a takrolimus at pregnancy is not established adequately, therapy by drug during this period is allowed only in the absence of safer alternative way of treatment, and only in case of considerable exceeding of advantage for mother over potential risk for a fruit.
According to clinical observations такролимус gets into breast milk thereof for the period of therapy by Advagraf breastfeeding should be interrupted.
At heavy hepatic dysfunction, perhaps, reduction of a dose of drug will be required.
At a renal failure there is no need of correction of doses of a takrolimus, but because of its nephrotoxic potential it is recommended to control carefully function of kidneys – concentration of serumal creatinine, the clearance of creatinine (CC) and amount of the emitted urine.
Route of administration and dosage
Capsules of the prolonged action Advagraf accept inside right after extraction from the blister, entirely, washing down with liquid (preferably water). The daily dose is recommended to be taken for 1 time, in the morning, on an empty stomach – for 1 h to food or in 2-3 h after meal (for achievement of the maximum absorption of a takrolimus). The passed dose is accepted as soon as possible, it is desirable on the same day, it is not necessary to accept a double dose the next morning.
It is necessary to warn patients about existence in packaging of a bag of silica gel for moisture absorption which is not intended for the use.
Reception duration Advagrafa is not limited as the condition of immunosuppression for prevention of graft rejection needs to be supported constantly.
The recommended dosing mode (1 time a day, in the mornings):
- Prevention of rejection of a transplantirovanny kidney: initial daily dose of 0,2-0,3 mg/kg; administration of drug needs to be begun during 24 h after transplantation;
- Prevention of rejection of a transplantirovanny liver: initial daily dose of 0,1-0,2 mg/kg; administration of drug needs to be begun in 12-18 h after transplantation;
- Transplantation of a kidney or liver: transition on Advagraf from other immunodepressants should be begun with the doses recommended for prevention of rejection of a transplantirovanny kidney or a liver;
- Transplantation of heart: transition on Advagraf from other immunodepressants it is necessary to begin 0,15 mg/kg with a dose;
- Transplantation of other bodies: there is no clinical experience of use of Advagraf in therapy of patients after change of a pancreas, a lung, intestines, but as a part of the drug Pro-columns такролимус is used after transplantation of a lung in an initial peroral dose of 0,1-0,15 mg/kg, a pancreas – 0,2 mg/kg, intestines – 0,3 mg/kg.
For stopping of graft rejection increase in a dose of a takrolimus, short courses of therapy by antibodies (моно-/polyclonal), therapy strengthening by glucocorticosteroids is recommended; in case of signs of toxicity of a takrolimus (the expressed undesirable side effects) Advagraf dose decline can be required.
Eventually after transplantation of a liver or a kidney of a dose of drug usually reduce, also transition to monotherapy with cancellation of the accompanying immunodepressants is possible. With improvement of a condition of the patient the pharmacokinetics of a takrolimus can change that demands additional correction of doses.
At the choice of doses of drug it is necessary to consider clinical assessment of individual portability of drug and probability of graft rejection, and also data of monitoring of concentration in blood of a takrolimus. In most cases, according to clinical trials, therapy is successful if this indicator ≥ 20 ng/ml.
Therapeutic concentration in blood of a takrolimus at the initial stage:
- Kidneys or heart (after transplantation) – 10-20 ng/ml;
- Liver (after transplantation) – 5-20 ng/ml;
- Liver, kidneys or heart (in the course of the supporting immunosuppressive therapy) – 5-15 ng/ml.
Correction of doses for achievement of equal minimum concentration of a takrolimus at separate categories of patients:
- Race – higher doses, than to patients of Caucasian race can be required by black patients;
- Paule – does not have data on need of different doses to men and women;
- Advanced age – there are no data on need of special doses.
Careful control at therapy by Advagraf from medical personnel of the corresponding qualification is required and existence of the necessary equipment. Drug can be appointed only by the doctor having experience of performing immunosuppressive therapy at patients with transplanted organs.
In case of clinical signs of rejection the question of need of correction of dosing of immunosuppressive therapy has to be considered.
Side effects
Precisely it is difficult to establish a profile of undesirable reactions of immunodepressants because of features of a basic disease and large amount of the medicines used after organ transplantation.
Most often (> 10%) the following side effects are noted: renal failure, tremor, hyperglycemia, diabetes mellitus, infections, hypertension, hyperpotassemia, sleeplessness.
At Advagraf's use in therapeutic doses the following side effects from bodies and systems are possible (distribution on frequency according to the following gradation: very often –> 1/10, it is frequent –> 1/100-<1/10, infrequently –> 1/1000-<1/100, is rare –> 1/10 000-<1/1000, is very rare – <1/10 000, uncertain frequency – there are not enough data for establishment):
- Infections and invasions: the probability of local and generalized infectious defeats is increased (bacterial, protozoan, virus, fungal); deterioration in a course of earlier diagnosed infections is possible; cases of the nephropathy associated with the VK-virus, and the progressing multifocal leukoencephalopathy associated with a JC virus were observed;
- The tumors (which are not specified, high-quality and malignant) including cysts and polyps: the risk of malignant new growths is increased; developing of both malignant, and benign tumors, including the limfoproliferativny diseases associated with Epstein-Barre's virus and a carcinoma cutaneum is noted;
- Blood and lymphatic system: often – thrombocytopenia, anemia, a leukopenia, a leukocytosis, aberrations in results of the analysis of erythrocytes; infrequently – a neutropenia, a pancytopenia, coagulopathies, deviations in data of a koagulogramma; seldom – a prothrombinopenia, a trombotichesky Werlhof's disease; uncertain frequency – an agranulocytosis, a partial krasnokletochny aplasia, hemolitic anemia;
- Immune system: anaphylactic and allergic reactions;
- Endocrine system: seldom – a hirsutism;
- Metabolism and food: very often – a hyperglycemia, a diabetes mellitus, a hyperpotassemia; often – a metabolic acidosis, a loss of appetite, anorexia, electrolytic disturbances, a hypophosphatemia, a hypervolemia, a hyponatremia, a hyperuricemia, a hypopotassemia, a hypomagnesiemia, a hypocalcemia, a lipidemia, a gipertriglitseridemiya, a hypercholesterolemia; infrequently – a hyperphosphatemia, a hypoglycemia, dehydration, a hypoproteinemia;
- Mental disorders: very often – an insomniya; often – a depression, uneasiness, confusion of consciousness and a disorientation, hallucinations, affective frustration, the suppressed mood, nightmares; infrequently – psychotic frustration;
- Nervous system: very often – a tremor, a headache; often – spasms, consciousness disturbances, dizziness, peripheral neuropathy, a dizesteziya and paresthesia, disturbance of the letter, frustration of a nervous system; infrequently – hemorrhages in TsNS and disturbance of cerebral circulation, encephalopathy, disturbances of an articulation and the speech, amnesia, paralysis and paresis, a coma; seldom – a hyper tone; very seldom – a myasthenia;
- Organ of sight: often – photophobia, an illegibility and a vision disorder; infrequently – a cataract; seldom – total loss of sight;
- Acoustic organ and vestibular disturbances: often – a ring and a sonitus; infrequently – a hearing impairment; seldom – neurosensory deafness; very seldom – a hearing disorder;
- Heart: often – tachycardia, ischemic coronary frustration; infrequently – tachycardia, ventricular arrhythmias (up to a cardiac standstill), heart failure, a cardiomyopathy, supraventricular arrhythmias, pathological changes on the electrocardiogram (ECG), a hypertrophy of ventricles, disturbances of pulse and the heart rate (HR); seldom – a pericardiac exudate; very seldom – ventricular takhisistolichesky arrhythmia on the pirouette type, lengthening on an ECG of an interval of QT, pathological changes on an ekhokardiogramma;
- Vessels: very often – arterial hypertension; often – vascular hypotension, ischemic and tromboembolic episodes, disturbance of peripheric circulation, bleeding; infrequently – a deep vein thrombosis of extremities, a heart attack, shock;
- Respiratory system, bodies of a thorax and mediastinum: often – short wind, cough, pulmonary parenchymatous frustration, a pleural exudate, a nose congestion, rhinitis, pharyngitis; infrequently – asthma, frustration from respiratory tracts, respiratory insufficiency; seldom – ARDS (acute respiratory distress syndrome);
- Digestive Tract (DT): very often – nausea, diarrhea; often – abdominal and gastrointestinal pain, vomiting, inflammations, bleedings, ulcers and perforations, ascites, stomatitis and an ulceration mucous a mouth, a lock, a liquid chair, a meteorism, dyspepsia, feeling of swelling and a raspiraniye in a stomach; infrequently – chronic and acute pancreatitis, increase in activity of amylase in blood, peritonitis, paralytic Ilheus (intestinal impassability), a gastroesophageal reflux, disturbance of evakuatorny function of a stomach; seldom – субилеус, pancreatic pseudocysts;
- Kidneys and urinary tract: very often – disturbance of work of kidneys; often – an acute and chronic renal failure, an acute canalicular necrosis, a toxic nephropathy, damage of urinary tract, disorders of function of a bladder and an urethra, an oliguria; infrequently – an anury, a hemolitic uraemic syndrome; very seldom – hemorrhagic cystitis, a nephropathy;
- Gepatobiliarny system: very often – pathological changes of results of functional hepatic tests; often – a cholestasia, pathologies of bilious ways, defeat of cells of a liver, hepatitis, jaundice; seldom – the thrombosis of a hepatic artery obliterating an endophlebitis of hepatic veins; very seldom – a liver failure;
- Skin and hypodermic fabrics: often – rash, an alopecia, a hyperhidrosis, an itch, an acne; infrequently – a photosensitization, dermatitis; seldom – a Lyell's disease (a toxic epidermal necrolysis); very seldom – Stephens-Johnson's syndrome;
- Skeletal and muscular and connecting fabric: often – a dorsodynia and extremities, an arthralgia, muscular spasms; infrequently – joint frustration;
- Generative organs and mammary gland: infrequently – uterine bleeding, a dysmenorrhea;
- General frustration: often – feverish states, an adynamy, pain and discomfort, disturbance of perception of body temperature, hypostases, increase in body weight, increase in activity of an alkaline phosphatase in blood; infrequently – a grippopodobny syndrome, decrease in body weight, increase in activity of a lactate dehydrogenase in blood, feeling sick, feeling of alarm, feeling of squeezing in a breast, multiorgan insufficiency, disturbance of perception of ambient temperature; seldom – feeling of constraint in a breast, thirst, falling owing to loss of balance, difficulty of the movement; very seldom – increase in mass of lipidic fabric;
- Injuries, complications of manipulations, intoxications: often – primary dysfunction of a transplant.
Many undesirable reactions are reversible and/or decrease at reduction of a dose of a takrolimus.
Mistakes in appointment and delivery of drugs of a takrolimus, including accidental, unreasonable or uncontrolled substitutions of one dosage form with another were noted, and also the related cases of graft rejection were registered (data for frequency assessment insufficiently).
Special instructions
Without implementation of appropriate control transfer of patients from one drug of a takrolimus on another (including on capsules of the prolonged action from ordinary capsules) is unsafe. Graft rejection or increase in frequency of side effects (including hypo - or hyper immunosuppression) because of clinically significant distinctions in exposure of a takrolimus can be a consequence of it. Change of the mode of dosing or a dosage form is carried out only under control of the doctor-transplantologist. After transfer careful monitoring of concentration of a takrolimus is carried out to blood and the drug dose for maintenance of system exposure of substance at the adequate level is adjusted.
At first after transplantation it is required to carry out regular monitoring of the following parameters: ECG, arterial pressure, neurologic status, condition of sight, concentration of electrolytes (especially potassium), concentration of glucose on an empty stomach, indicators of function of kidneys and liver, hematologic indicators, koagulogramma, existence in plasma of proteins. In case of clinically significant changes it is recommended to carry out correction of immunosuppressive therapy.
At diarrhea perhaps considerable change of concentration of a takrolimus in blood therefore at the speeded-up defecation it is necessary to control this indicator carefully.
As ink for marking of capsules of Advagraf contains soy lecithin, with hypersensitivity to soy or a peanut it is important to patients to compare risk of an allergy with advantage of use of a takrolimus.
Takrolimus, especially in combination with ethanol, is capable to cause neurologic and visual frustration that should be considered when performing types of the works demanding the increased concentration of attention and speed of psychomotor reactions.
Medicinal interaction
- Drugs or officinal herbs with the inducing or inhibiting effect on CYP3A4 – can raise or lower concentration of a takrolimus respectively (for maintenance of its stable adequate exposure it is recommended to control concentration and, in case of need, to adjust a dose or to stop reception; control of function of kidneys and possible side effects is also necessary);
- Antifungal means (флуконазол, кетоконазол, вориконазол, итраконазол), protease HIV inhibitors (ритонавир, саквинавир, нелфинавир), makrolidny antibiotics (erythromycin), inhibitors of proteases of a virus of hepatitis C (боцепревир, телапревир) – are capable to increase significantly concentration of a takrolimus (in case of need their simultaneous use decrease in doses of Advagraf almost at all patients can be required);
- Clotrimazolum, кларитромицин, джозамицин, nifedipine, никардипин, diltiazem, verapamil, Amiodaronum, даназол, ethinylestradiol, омепразол and нефазодон – medicinal interaction with takrolimusy is less expressed;
- The cortisone, Bromocriptinum, dapsone, ergotamine, lidocaine, Mephenytoinum, гестоден, Miconazolum, midazolam, Norethisteronum, quinidine, Tamoxifenum, triatsetit (Oleandomycinum) – potential inhibitors of metabolism of a takrolimus (research in vitro);
- Grapefruit juice – can increase concentration of a takrolimus;
- Lansoprazol and cyclosporine – are potentially capable to inhibit SURZA4, indirectly increasing the level of a takrolimus;
- The drugs having high affinity to proteins of a blood plasma (peroral anticoagulants, non-steroidal anti-inflammatory drugs (NPVP), peroral anti-diabetics) – their possible competitive interaction with takrolimusy is required to consider;
- Phenytoinum, rifampicin, St. John's Wort made a hole (Hypericum perforatum) – can reduce considerably concentration of a takrolimus (data of clinical experiences) (when sharing need of increase in a dose of Advagraf is probable);
- Phenobarbital – clinically significant interactions were observed;
- Corticosteroids (maintenance doses) – usually reduce concentration of a takrolimus;
- Prednisolonum or Methylprednisolonum (in high doses) – are capable to reduce or increase the level of a takrolimus;
- Carbamazepine, metamizol and isoniazid – can reduce concentration of a takrolimus;
- Cyclosporine – increases its elimination half-life of T1/2, nephrotoxic synergy/additive effects are possible (their concomitant use is not recommended, and at purpose of a takrolimus the patients accepting cyclosporine earlier should be careful);
- Phenytoinum – такролимус increases its concentration in blood;
- Hormonal contraceptives – because of ability of a takrolimus to reduce their clearance it is important to choose contraceptives carefully;
- Statines – their pharmacokinetics does not change (data of clinical observations);
- Phenobarbital and antipyrine – decrease in their clearance and increase in T1/2 is potentially possible (data of experiments on animals);
- Prokinetics (цизаприд, Metoclopramidum), magnesium hydroxide and aluminum, Cimetidinum – are capable to increase system exposure of a takrolimus;
- Aminoglycosides, Vancomycinum, giraza inhibitors, co-trimoxazole, NPVP, acyclovir, ганцикловир – strengthening nefro-or a neurotoxicity is possible;
- Amphotericinum In and an ibuprofen – amplifies their nephrotoxicity;
- Potassium or kaliysberegayushchy diuretics (Triamterenum, amiloride, Spironolactonum) – development or strengthening of a hyperpotassemia is possible (it is necessary to avoid their use in high doses);
- Vaccines, especially live weakened – такролимус can change reaction of an organism to vaccination, reducing its efficiency (it is necessary to avoid vaccination).
Test tubes, syringes and other equipment which is used at preparation of suspension from the powder which is contained in capsules Advagraf should not contain polyvinylchloride (PVC) as такролимус with it it is incompatible.
Terms and storage conditions
To store at a temperature up to 25 °C in original packaging. To protect from children.
Period of validity – 3 years. After opening of an aluminum package drug is stored 1 year.
Name of drug
Price
Drugstore
Caries is the most widespread infectious disease in the world to which even flu cannot compete.

Olive oil – the product capable to make a powerful contribution to health of the person if it includes it in the diet. Rich vitamin...
Section: Articles about health
Hemorrhoids – extremely widespread disease. Periodically arising inflammations and bleeding of hemorrhoidal nodes cause serious discomfort to nearly fifteen percent of adults. Meanwhile, having a clear idea of the aggravation reasons...
Section: Articles about health
Physical activity is necessary for normal functioning of a human body. At a lack of the movement joints cease to function, muscles atrophy, cardiovascular activity is broken and the metabolism worsens. The modern city rhythm of life does not provide the person with an adequate exercise stress, additional - sport is necessary. Tedious tasks the huge number of the people having those or ин exists sport not all to liking, but also...
Section: Articles about health
The medicine promptly develops, and the fact that else quite recently it seemed by miracle can now. We are not surprised any more to the fact that sport...
Section: Articles about health
New year, wedding, birthday, office party – an occasion to drink at the Russian person will always be. How to reduce a negative impact of alcohol by an organism and to avoid a condition of strong intoxication? The most correct council – to refuse the use spirits напитк...
Section: Articles about health
Is told about advantage of domestic animals for development of the child much. But many parents nevertheless do not hurry to bring pets as are afraid that they can do harm to health of children. What troubles can really trap kids and how to make joint life of a family and domestic animals comfortable and safe?...
Section: Articles about health
The pine is one of the most widespread plants of our woods. Its needles and pitch not without reason called by "gallipot", since ancient times испол...
Section: Articles about health
Bees – really unique beings. Practically all products of their life activity are used by the person. Since the most ancient times medicinal properties of honey and other substances received in the course of beekeeping are known. The fact that all these пр is especially significant...
Section: Articles about health
A lot of things depend on a condition of a backbone in a human body, a backbone - not only a support for a body, it also a receptacle for a spinal cord, that is why malfunctions with a backbone are so dangerous. To treat rachis diseases very difficult and long, it is much simpler and more correct not to bring to a disease. Conforming to the rules provided in this article it is possible to avoid the majority of the problems connected with a backbone including those which are considered to be age, but a cat...
Section: Articles about health
For most of the working people the problem of having a snack is particularly acute enough. Sooner or later there is a question: what is possible quickly for a sja...
Section: Articles about health
Each woman has preferences in the field of use of those goods which help us to look good, feel young and effective. Besides: selection process of favourite perfume, shampoo or decorative cosmetics already lifts a spirit...
Section: Articles about health
There is an opinion that at low temperatures safety of products is ensured longer and better thanks to what the refrigerator is considered the most suitable place for storage of food. In most cases it is fair, however there is a number of products for which low temperatures – the main reason of their premature damage. Storage in the refrigerator leads to their bystry rotting, emergence of a mold, is followed by loss of vitamins and tastes. What products it is better to remove...
Section: Articles about health
More than a half of the married couples which faced prostatitis – leave. The new broadcast "Female View of Prostatitis" will help to learn...
Section: Articles about health
Diapers for adults – individual one-time means of hygiene which in some situations is irreplaceable and from such situations any person is not insured. Though nobody perceives need of their use with enthusiasm, however without it to a sra...
Section: Articles about health
The way of life of people promptly changes from year to year: if about ten years ago the personal computer was not in each family, then today already very few people do without this device. Certainly, and children master the computer at full speed: they not only play on it games, but also study, and write school works, and search for necessary information....
Section: Articles about health
Health and attractiveness - eternal values, pursuing which people often use the most unusual ingredients and technicians...
Section: Articles about health
The next flu epidemic leads to the next panic, from year to year we give in on these manipulations: professionally alarming voice of the announcer in news, reports with calculation of the died patients, an interview with people in white dressing gowns and advertizing of anti-influenza means ра...
Section: Articles about health
The kid who was recently born is surrounded with love of adult family members and their cares without which the baby cannot exist. Some parents consider that gentle attachment and caress are quite enough that the child correctly developed and was happy, but it not so. It is important to know as much as possible about specifics of care of the baby, the reasons of his behavior and possible problems. Only the "able to see" love will provide to the little man that it is necessary for him....
Section: Articles about health
Eyes – unique body on the structure thanks to which the person obtains about 80% of information on the world around: about a form...
Section: Articles about health
Cold is such painful that each sigh becomes a victory, heat "knocks" down, and the ache in joints forces to think only of pain. Some people with approach of the first symptoms of cold make the self-sacrificing decision to have a disease standing, and to a beam...
Section: Articles about health
The modern person not always manages to find housing in the environmentally friendly region and such work which would not do harm to health. With food stuffs at first sight the situation is much better: shops are overflowed with goods which are positioned by producers as very useful and absolutely safe. Many Russians are absolutely sure that the choice of products with marking "bio", "эко" or "organik" guarantees them and members of their families an optimal variant of food. To a sozhala...
Section: Articles about health
Subfebrile temperature call fervescence to 38 degrees, and subfebrile condition - existence of such temperature from above...
Section: Articles about health
All are familiar with cold, and practically everyone believes that he has sufficient knowledge and experience that correctly to treat it. In practice most of people makes mistakes in attempts to get rid of rhinitis, and divides numerous delusions it....
Section: Articles about health
Producers of milk mixes for children assure: mixes are ideally balanced and adapted for needs of babies. If mother should raise artificially the kid owing to serious problems with health, to do nothing – it is necessary to feed with substitutes of milk. However pediatricians note that not seldom women without good reasons refuse feeding of the child a breast and pass to milk mixes. Common causes of such decision – the aspiration to leave quicker...
Section: Articles about health
It is difficult to revaluate importance of kidneys for an organism. These bodies not only perform work on purification of blood of decomposition products and выв...
Section: Articles about health
For the city dweller the fitness is the most convenient sport. It is enough to acquire the subscription to the gym to get access to various apparatuses and an opportunity to train under the leadership of the experienced consultant. Many consider fitness on...
Section: Articles about health
Eyes – one of the most vulnerable areas on a face therefore age changes concern them first of all. Whether it is possible to keep look youth for many years and what procedures are offered for achievement of this purpose by cosmetologists? And maybe, the only option of rejuvenation is surgery – a blepharoplasty? Let's try to understand this question....
Section: Articles about health

