The benzoic acid
The benzoic acid (e210) – the preservative used in the food industry.
Description and characteristic
For the first time 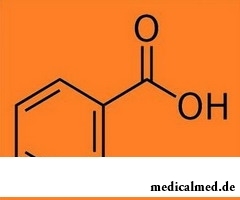 acid was emitted in the 16th century by means of a method of sublimation of the benzoin resin. In the 19th century Germans could define structure of acid, investigated its properties and compared its characteristics to hippuric acid. As a result in the second half of the 19th century antimicrobic effect of the benzoic acid was revealed. And in the 20th century of its steel widely to apply to conservation of foodstuff.
acid was emitted in the 16th century by means of a method of sublimation of the benzoin resin. In the 19th century Germans could define structure of acid, investigated its properties and compared its characteristics to hippuric acid. As a result in the second half of the 19th century antimicrobic effect of the benzoic acid was revealed. And in the 20th century of its steel widely to apply to conservation of foodstuff.
On the physical properties the benzoic acid represents needles or brilliant monoklinny leaflets of white color which melt at a temperature of 122 degrees Celsius. Acid is well dissolved in water, fats and anhydrous alcohol.
From the chemical point of view preservative can be carried to monobasic carboxylic acids of an aromatic series. E210 – natural substance which contains in a number of berries: cranberry, bilberry, cowberry. There is it in honey in the connected look. The benzoic acid is formed in such dairy fermented products as yogurt or curdled milk, as a result of microbic decomposition of hippuric acid. Also it contains in some essential oils, for example, in clove. Antimicrobic properties of the benzoic acid are based on oppression of activity of enzymes in cells of microbes.
In the synthetic way acid is received during toluene oxidation. At the moment this way of receiving acid is most widespread and it is considered the most favorable as raw materials for this purpose inexpensive and process do not make negative impact on the environment.
Earlier the benzoic acid was received also by means of acid hydrolysis of phenyl chloroform and decarboxylation of phthalic acid with influence of catalysts. But now such way of receiving acid is not urgent.
Use of the benzoic acid
The food industry of property of the benzoic acid is applied in the confectionery, brewing and baking industry. Use it in preparation of margarine, jam, fruit juice, a vegetable pickles, marinated fish, milk products, chewing gums, ice cream, seasonings, liqueurs, candies and substitutes of sugar.
Besides,  along with ethers and salts use e210 and in the cosmetic industry. In the form of benzyl benzoate it is applied in pharmaceutics (add to ointments against itch).
along with ethers and salts use e210 and in the cosmetic industry. In the form of benzyl benzoate it is applied in pharmaceutics (add to ointments against itch).
In the medical purposes acid is used as fungicidal and antimicrobic means. It is added to many cough medicine as it plays a role of an antiseptic agent and has expectorant effect. Well additive e210 proved at treatment of perspiration of legs and fungus diseases of skin.
Widely use the benzoic acid in chemical industry. So, when synthesizing many organic matters acid often plays a role of the main reagent.
Influence of the benzoic acid on a human body
Preservative e210 in general is well acquired by a human body and interacts with proteinaceous connections, forming hippuric acid in the form of which the organism also brings him kidneys.
According to some information e210 can enter interaction with ascorbic acid, forming strong carcinogen – free benzene. Therefore it is necessary to avoid products as a part of which there is an ascorbic acid and additive e210.
In Russia there is strictly certain dosage for preservative e210 in food stuffs. Its quantity should not exceed 5mg/kg, otherwise acid negatively influences a condition of kidneys and a liver.
Four segments of dark chocolate contain about two hundred calories. So if you do not want to recover, better not to eat it is more than two segments in days.

Olive oil – the product capable to make a powerful contribution to health of the person if it includes it in the diet. Rich vitamin...
Section: Articles about health
The metabolism at each person proceeds in own way. However between the speed of this process and disposal of excess weight after all all have a dependence. Unfortunately, the people inclined to try on itself numerous "miracle" diets, not always at...
Section: Articles about health
Iodine - one of thirty most important microelements in our organism. The main role of iodine consists in synthesis of thyroid hormones of a thyroid gland - the substances which are responsible for the majority of exchange processes of an organism. It is known that thyroid hormones consist of iodine more than for 65%. The lack of iodine leads to decrease in production of hormones and, as a result, development of a hypothyroidism. The long condition of deficit can become a source of problems of the cardiovascular, bone, digestive SI...
Section: Articles about health
Good appetite was always considered as a sign of good health. The correct operation of the mechanism which is responsible for the need for nutritious...
Section: Articles about health
Each failure in work of bodies and systems of a human body is, as a rule, shown by the whole complex of symptoms. In particular, malfunctions with health often cause emergence of cosmetic defects in the form of rashes on a face. Experienced doctors know that локализац...
Section: Articles about health
Work of a brain is extremely complex and in many respects is not studied yet. It is confirmed also by the features of thought processes which are shown when the person sleeps. Let's tell about some of them....
Section: Articles about health
Helminthosis is one of the most widespread diseases. Statistically, with any species of helminths it is infected porridges...
Section: Articles about health
So, you resolved to lose weight. And now you try to understand what to begin with: from exercise stresses or a diet? And how to make that process of weight loss did not give you an inconvenience, and, on the contrary, brought joy?...
Section: Slideshow
History of use of an anesthesia during operations contains more than 160 years. Annually in the world hundreds of thousands of surgical interventions during which to patients the substances immersing them in a dream and saving from pain are entered are carried out. Using an anesthesia many myths and delusions are still connected. It is worth getting acquainted with the most widespread of them....
Section: Articles about health
Partial and the more so full loss of hearing significantly reduces quality of life. Difficulties with communication lead to loneliness and замкн...
Section: Articles about health
What is in our understanding weeds? It plants which are considered to be suitable only for compost pits and feeding of animals. Meanwhile, among the weeds growing literally under legs it is possible to find the mass of the officinal herbs possessing invaluable Paul...
Section: Articles about health
The cosmetics intended for improvement of a condition of skin, nails and hair are used by each woman. Expenses on regular acquisition of the fashionable widely advertized products of well-known companies for many become very notable and significantly burden the family budget. Meanwhile, there is a number of inexpensive pharmaceutical drugs which can quite be applied in the cosmetic purposes. At the same time the effect of their use is often more noticeable, than result of use of the most expensive...
Section: Articles about health
Not everyone can brag of the shining Hollywood smile. Even at the person who is regularly visiting the stomatologist and watching з...
Section: Articles about health
For the person who daily since morning gathers for work it is very important to wake up vigorous and ready by day of work. Actually, each of us experiences difficulties with this, at first sight, simple business from time to time. On a condition of an organism after ночн...
Section: Articles about health
Many of us, probably, noticed more than once that from intellectual loadings at some point the brain as though "overheats" and "assimilation" of information is strongly slowed down. Especially this problem urgent for persons of age becomes more senior than fifty years. "Already badly I think", "the head will burst now", "memory as if is disconnected" - here that wants to be told at the time of information overload....
Section: Articles about health
Statistically, can only one of ten of our compatriots brag of a decent condition of an oral cavity. On среднестатистич...
Section: Articles about health
Color of plants is caused by presence at them of certain chemical compounds. Let's talk about what is meant by various colors of vegetables and fruit and what properties they give them....
Section: Articles about health
Tick-borne encephalitis – one of the most dangerous viral diseases which causative agents transfer and is given to people by ixodic mites. These are the small blood-sicking insects living in the considerable territory of our country. The person bitten by a tick can catch also erlikhiozy, bartonnelezy, babeziozy, mycoplasmosis and Lyme's disease. As well as encephalitis, these illnesses affect the central nervous system, and as specific antiviral therapy does not exist, the forecast very to a neuta...
Section: Articles about health
The state of health of the person in many respects depends on food. The organism will well function if during food it are...
Section: Articles about health
A lot of things depend on a condition of a backbone in a human body, a backbone - not only a support for a body, it also a receptacle for a spinal cord, that is why malfunctions with a backbone are so dangerous. To treat rachis diseases very difficult and long...
Section: Articles about health
According to World Health Organization, every third inhabitant of Earth has excess weight, and every tenth has obesity. The reason of this phenomenon, according to specialists, roots in one not very comforting fact: most of people consume much more calories, than it is necessary. How it turns out what we overeat? Why it is so difficult to refuse an excess portion tasty or additives? Let's try to find out what factors prevent us to eat food with reasonable moderation....
Section: Articles about health
The thought that the mass of their body is too big at least once in life visits from 80 to 95% of women. Many...
Section: Articles about health
Long time antibiotics were considered as a panacea from all diseases and were appointed even at insignificant symptoms of an infection. Even now not everyone knows in what force of antibiotics how and when they should be accepted. Let's discredit 7 popular myths about such drugs...
Section: Articles about health
Ayurveda - the most ancient tselitelsky practice which came to us from India. It represents the doctrine about maintenance of physical, psychological and moral health of the person by means of the complex of procedures including a diet, cleaning of an organism, breathing exercises, massage, and in case of a disease - and medicinal therapy. The healers practicing Ayurveda assign very important part to spices, and at the heart of Ayurvedic drugs, as a rule, there are they. It is considered that spices not of t...
Section: Articles about health
Ability of an organism to resist to adverse environmental factors (to impact of temperature drops, humidity and pressure...
Section: Articles about health
The sudden heat on all body which is followed by perspiration and a cardiopalmus – the phenomenon familiar to many people. Most often such states called by "inflows" result from nervous or physical overworks and disappear right after rest. Odn...
Section: Articles about health
The trophic ulcer is not an independent disease. This heavy complication arising owing to a thermal injury (a burn or a frostbite), chronic pathologies of arteries or veins of the lower extremities, a diabetes mellitus, and also some defeats of connecting fabric, absorbent vessels, skin or nervous trunks. Pathology is shown in the form of not healing wound located on the internal surface of a shin, a foot sole, a heel or toes....
Section: Articles about health