





Ko-Renitek
Application instruction:
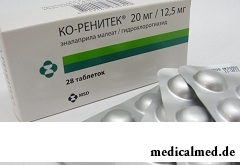 Ko-Renitek – drug with hypotensive and diuretic action.
Ko-Renitek – drug with hypotensive and diuretic action.
Form of release and structure
Ko-Renitek is let out in the form of tablets: yellow, biconvex, round, with corrugated edge, on one party – an engraving of "MSD 718", on another – risk (in blisters on 7 or 14 pieces, on 1, 2 or 4 blisters in a cardboard pack; in polyethylene bottles on 56 pieces, on 1 bottle in a cardboard pack).
Is a part of 1 tablet:
- Enalapril maleate – 20 mg;
- Hydrochlorothiazide – 12,5 mg.
Auxiliary components: dye ferrous oxide yellow, sodium bicarbonate, lactose water (monohydrate of lactose), prezhelatinizirovanny corn starch, corn starch, magnesium stearate.
Indications to use
Ko-Renitek is appointed at arterial hypertension in the presence of indications to carrying out a combination therapy.
Contraindications
- Anury;
- Quincke's disease (idiopathic, hereditary or existence of instructions in the anamnesis on its development at the previous use of inhibitors of an angiotensin-converting enzyme);
- Hypersensitivity to drug components, and also to other derivatives of sulfonamide.
Efficiency and Ko-Renitek's safety at children are not established in this connection use of drug in pediatrics is not recommended.
Ko-Renitek do not appoint sick with a renal failure which are on a hemodialysis.
Ko-Renitek's use by pregnant women is not recommended, during a lactation at purpose of drug it is necessary to interrupt breastfeeding.
Ko-Renitek needs to apply with care to elderly patients, at states after transplantation of a kidney, against the background of a diet with sodium restriction, at states which are followed by decrease in volume of the circulating blood (including diarrhea and vomiting), and also the patient with an aortal stenosis, cerebrovascular diseases (including insufficiency of cerebral circulation), coronary heart disease, chronic heart failure, serious autoimmune system illnesses of connecting fabric (including a system lupus erythematosus and a scleroderma), oppression of a marrowy hemopoiesis, a diabetes mellitus, a hyperpotassemia, a bilateral stenosis of renal arteries, a stenosis of an artery of the only kidney, a renal and/or liver failure.
Route of administration and dosage
Ko-Renitek is accepted inside, regardless of meal.
Initial daily dose at arterial hypertension – 1 tablet, further, if necessary, its increase is possible twice (in 1 reception).
At the beginning of therapy there can be symptomatic arterial hypotension, is preferential at disturbances of water and electrolytic balance because of the previous treatment by diuretics (in 2-3 days prior to Ko-Renitek's reception therapy by diuretics needs to be stopped).
At functional disturbances of kidneys of a tiazida can be insufficiently effective, and at clearance of creatinine of ≤30 ml a minute (i.e. at heavy and average degree of a renal failure) are inefficient.
At clearance of creatinine of 30-80 ml a minute Ko-Renitek can be accepted only after individual selection of a dose of each of components. At easy degree of a renal failure the recommended dose of the maleate of enalapril accepted separately makes 5-10 mg.
Side effects
At conduct of clinical trials side effects, as a rule, were moderate, passing and in most cases interruptions of therapy did not demand. During Ko-Renitek's use there can be following disturbances (> 1-2% – often; 1-2% – infrequently; <1-2% – are rare):
- Central and peripheral nervous system: often – increased fatigue (usually passes at reduction of a dose, demands drug withdrawal seldom), dizziness; infrequently – headaches, an adynamy; seldom – drowsiness, sleeplessness, paresthesias, a rotatory vertigo, a hyperexcitability;
- Cardiovascular system: infrequently – orthostatic effects, including arterial hypotension; seldom – tachycardia, a faint, arterial hypotension regardless of position of a body, a stethalgia, heartbeat;
- Alimentary system: infrequently – nausea; seldom – diarrhea, pancreatitis, dyspepsia, vomiting, abdominal pains, a lock, a meteorism, dryness in a mouth;
- Reproductive system: infrequently – impotence; seldom – decrease in a libido;
- Urinary system: seldom – functional disturbances of kidneys, a renal failure;
- Respiratory system: infrequently – cough; seldom – an asthma;
- Musculoskeletal system: infrequently – muscular spasms; seldom – an arthralgia;
- Laboratory indicators: a hyperglycemia, a hyperuricemia, hyper - or a hypopotassemia, increase in concentration in blood of serumal creatinine and urea, increase in serumal bilirubin and/or activity of liver enzymes (these indicators usually are returned to norm after drug withdrawal); in some cases – decrease in a hematocrit and hemoglobin;
- Dermatological reactions: seldom – a hyperhidrosis, Stephens-Johnson's syndrome, an itch, skin rash;
- Allergic reactions: seldom – a Quincke's disease of extremities, persons, language, lips, a throat and/or a glottis. There are rare messages on emergence of a Quincke's disease of intestines because of reception of inhibitors of an angiotensin-converting enzyme, including enalapril;
- Others: seldom – gout, a sonitus. The symptom complex which possible manifestations is the arthritis/arthralgia, fever, a vasculitis, a serositis, a miositis, a mialgiya, positive test for antinuclear antibodies, acceleration of a blood sedimentation rate, a leukocytosis, an eosinophilia is described; development of a photosensitization is possible.
Special instructions
During Ko-Renitek's use there can be symptomatic hypertensia. It is necessary to control clinical signs of disturbances of water and electrolytic balance, including organism dehydration, a gipokhloremichesky alkalosis, a hyponatremia, a hypomagnesiemia or a hypopotassemia which can arise because of episodes of vomiting or diarrhea. At such patients during therapy periodically through certain periods it is necessary to define electrolytic composition of blood.
With extra care of Ko-Renitek appoint at coronary heart disease or cerebrovascular diseases as excessive lowering of arterial pressure can lead to development of a stroke or myocardial infarction.
In cases of developing of arterial hypotension observance of a bed rest and, if necessary, intravenous administration of normal saline solution is shown. At Ko-Renitek's appointment passing arterial hypotension a contraindication to its further use is not. After normalization of arterial pressure and volume of the circulating blood treatment can be resumed or in a little reduced doses, or accepting each of drug components separately.
At a renal failure (clearance of creatinine <80 ml a minute) it is not necessary to appoint Ko-Renitek until selection of its separate components does not show that for this patient there are necessary doses at this dosage form.
Some patients without any symptoms of a disease of kidneys prior to therapy at use of enalapril in combination with diuretic can have an insignificant and passing increase in content of creatinine in serum and urea in blood. In such cases therapy is stopped. Treatment resuming is possible further or in a little reduced doses, or accepting each of drug components separately.
As well as other drugs with vazodilatiruyushchy action, Ko-Renitek needs to take with caution to patients at whom outflow of blood from a left ventricle of heart is complicated.
Sometimes at a bilateral stenosis of renal arteries or a stenosis of an artery of the only kidney at Ko-Renitek's use increase in content of creatinine in serum and urea in blood is observed. As a rule, these changes have reversible character, and after the treatment termination indicators are returned to norm.
It is necessary to apply thiazide diuretics at patients with the progressing diseases of a liver or disturbance of its functions with care as even minor changes of water and electrolytic balance can lead to development of a hepatic coma.
When carrying out big surgeries or during the general anesthesia with use of the drugs causing arterial hypotension, enalaprilat can block formation of angiotensin II which is caused by compensatory release of a renin. If at the same time there is expressed arterial hypotension which can be caused by the similar mechanism, it can be adjusted by increase in volume of the circulating blood.
Ko-Renitek can lead to development of disturbance of tolerance to glucose. In this case usually adjust doses of hypoglycemic medicines, including insulin.
Ko-Renitek can reduce calcium excretion with urine, and also is insignificant and is passing to increase the content of calcium in serum. The expressed hypercalcemia can be a symptom of the hidden hyperparathyreosis. Before carrying out a research of function of epithelial bodies reception of tiazid needs to be interrupted.
Increase in levels of cholesterol and triglycerides can be also connected using thiazide diuretics, however at a dose of a hydrochlorothiazide of 12,5 mg similar effects usually either are not observed, or are insignificant.
At some patients use of tiazid can lead to development of a hyperuricemia and/or gout. However enalapril can increase the content in urine of uric acid and weaken, thereby, giperurikemichesky effect of a hydrochlorothiazide.
At use of a maleate of enalapril exceptional cases of a Quincke's disease of extremities, persons, language, lips, a throat and/or a glottis were described. These disturbances can develop on any of therapy stages. In such cases it is necessary to interrupt immediately Ko-Renitek's reception and to watch carefully a condition of the patient for control and correction of clinical signs. Even if only the paraglossa without hypostasis of respiratory bodies is observed, long observation as uses of antihistamines and corticosteroids can be insufficiently can be required by patients.
In cases when hypostasis is localized in the field of language, a throat or a glottis that can cause obstruction of respiratory tracts, it is necessary to enter into short terms subcutaneously 0,3-0,5 ml of 0,1% of solution of adrenaline (Epinephrinum) and to provide passability of respiratory tracts.
At the patients of negroid race accepting inhibitors of an angiotensin-converting enzyme, the Quincke's disease was observed more often, than at other patients.
In the presence of instructions in the anamnesis the risk of development of a Quincke's disease significantly increases by a Quincke's disease which is not connected with reception of inhibitors of an angiotensin-converting enzyme against the background of performing therapy.
At the patients receiving tiazida allergic reactions can develop irrespective of existence in the anamnesis of bronchial asthma or allergic states. The patients receiving tiazida have messages on aggravation of weight of a current or a recurrence of a system lupus erythematosus.
In rare instances at the patients receiving inhibitors of an angiotensin-converting enzyme cases of development of life-threatening anaphylactoid reactions were noted during desensitization by allergen from poison of Hymenoptera. Such disturbances can be avoided if temporarily prior to carrying out desensitization to interrupt Ko-Renitek's reception.
At use of inhibitors of an angiotensin-converting enzyme cases of developing of cough were noted. Usually cough dry, has constant character and passes after the end of therapy (it is necessary to consider when carrying out differential diagnosis).
Medicinal interaction
At simultaneous use of Ko-Renitek with some medicines there can be following undesirable effects:
- Other hypotensive drugs: effect summation;
- Potassium additives, kaliysberegayushchy diuretics or kaliysoderzhashchy salts (in particular at patients with a renal failure): considerable increase of content of potassium in serum;
- Lithium drugs: lithium removal reduction by kidneys and strengthening of risk of development of intoxication by lithium;
- Non-steroidal anti-inflammatory drugs, including the selection TsOG-2 inhibitors, ethanol, estrogen: reduction of hypotensive effect of Ko-Renitek;
- Allopyrinolum, immunodepressants, cytostatics: increase in risk of development of a gematotoksichnost;
- Thiazide diuretics: strengthening of effect of tubocurarine.
Terms and storage conditions
To store in the place, unavailable to children, at a temperature up to 30 °C.
Period of validity – 2 years (for tablets in bottles of high density) or 3 years (for tablets in blisters).
Name of drug
Price
Drugstore
Co-renitek тбл 20/12.5mg No. 28, Merck Sharp & Dohme
515 rub.
 Network of the Moscow drugstores of IFC
Network of the Moscow drugstores of IFCMany drugs initially moved ahead in the market as drugs. Heroin, for example, was initially brought to the market as children's cough medicine. And cocaine was recommended by doctors as anesthesia and as the means increasing endurance.

The pine is one of the most widespread plants of our woods. Its needles and pitch not without reason called by "gallipot", since ancient times испол...
Section: Articles about health
Today about 30 diseases, sexually transmitted are known. Wide circulation of these illnesses is extremely promoted by the dual attitude towards them: on the one hand, most of people know about "shameful" diseases and not a stirrup very little...
Section: Articles about health
You heard that laughter prolongs life? Researchers did not manage to establish longevity direct link with sincere fun yet, but several facts confirming beneficial influence of risibility on the state of health are clinically proved....
Section: Articles about health
Eyes – one of the most vulnerable areas on a face therefore age changes concern them first of all. Whether it is possible to keep a pier...
Section: Articles about health
For the last decades the diabetes mellitus of the second type became really world problem. The number of cases annually increases, and average age of patients for whom the illness is diagnosed, steadily decreases. Specialists consider that one of osno...
Section: Articles about health
Statistically, can only one of ten of our compatriots brag of a decent condition of an oral cavity. Six teeth affected with caries are the share of the average Russian. For comparison, this indicator for Europeans is almost six times less....
Section: Articles about health
Life does not indulge the modern woman special emotional comfort and carelessness. Fatigue, troubles at work, misunderstanding...
Section: Articles about health
The unpleasant feelings connected with spring breakdown are familiar almost to each of us. Often happens that in March-April on the person weakness leans: he suffers from drowsiness, complains of bad mood, loss of interest in life and failures in affairs....
Section: Articles about health
Heart disease and blood vessels lead to disturbance of blood supply of bodies and fabrics that involves failures in their work, deterioration in health of the person, decrease in its working capacity and standard of living. Annually more than 17 million inhabitants of our planet perish from pathologies such....
Section: Articles about health
Epilepsy is one of widespread neurologic diseases. To parents, whose children suffer from this illness, it is necessary...
Section: Articles about health
Visit of doctors – business not the most pleasant, and many people do not hurry to undergo necessary planned inspections. Such behavior is extremely thoughtless and improvident. Our health is necessary not only to us: wellbeing of darlings, children, grandsons and престар...
Section: Articles about health
Dark circles (bruises) under eyes – a shortcoming with most of which often fight against the help of cosmetics (proofreaders, saloon procedures and so forth), eliminating only its visibility. However, according to doctors, skin around eyes – the indicator of many disturbances in an organism. To reveal them at early stages, without having disguised bruise, and having addressed its reasons – a task of each person who is regularly finding under with own eyes dark stains. Early detection and elimination of the disease lying in wasps...
Section: Articles about health
What they, women? Beautiful, gentle, passionate and at the same time windy, gusty, and nervous. And what is stranger: all эт...
Section: Articles about health
Olive oil – the product capable to make a powerful contribution to health of the person if it includes it in the diet. The rich vitamin composition of oil does it by a product number one from many diseases including from deadly. Only two tablespoons...
Section: Articles about health
Life activity of one-celled fungi of the sort Candida, related to yeast is a proximate cause of development of candidiasis (milkwoman). Normal these microorganisms are a part of the microflora living in an oral cavity and intestines of most of people and also in a female genital tract. The pathological phenomena are observed when fungi begin to breed too violently. At the same time there is an inflammatory process affecting mucous membranes and which is shown very nepr...
Section: Articles about health
Is told about advantage of domestic animals for development of the child much. But many parents nevertheless do not hurry to bring pets as about...
Section: Articles about health
Proofs of efficiency of Mildronate at treatment of coronary heart disease with stenocardia can be found in many publications of the end of the twentieth century. Researches were conducted since 1984, including placebo - controlled effects. In total клиничес...
Section: Articles about health
The concept "gluten" (differently, a gluten) combines group of the proteins which are a part of rye, barley and wheat. For most of people the use of the food stuffs containing a gluten not only is safe, but also it is very useful. Nevertheless, there is a number of myths about negative effect which allegedly gluten has on health of the person....
Section: Articles about health
The majority of gynecologic diseases prove three main signs, each of which speaks about need to the visa...
Section: Articles about health
Run - one of the most available and effective ways to revitalize the organism. Knowing about its extraordinary advantage, each of us at least once tried to make jogs, but only the few made these occupations regular. In spite of the fact that in jogging (easy an ozdor...
Section: Articles about health
The sudden heat on all body which is followed by perspiration and a cardiopalmus – the phenomenon familiar to many people. Most often such states called by "inflows" result from nervous or physical overworks and disappear right after rest. However in certain cases similar reaction of an organism can speak about diseases which need treatment. What? About it below....
Section: Articles about health
The brain of the person is studied not one hundred years, but the quantity of the riddles connected with this body increases rather, than reducing...
Section: Articles about health
Each person knows that fervescence is an illness sign. However too low temperature (hypothermia), especially also can demonstrate existence of diseases when it is observed long enough. Such state is dangerous those...
Section: Articles about health
Zone hypostases under eyes - very widespread problem giving to people is a lot of inconvenience. Hypodermic fabric in these parts has very loose structure and almost does not contain collagenic fibers. Besides, the skin covering подглазья constantly is exposed to compression and stretching when the person blinks, blinks, etc. These features also create premises for emergence of the so-called bags which are giving to the face a tired and sickly look, and also visually adding increased...
Section: Articles about health
Scientists have no unambiguous opinion on a proximate cause of emergence of a carcinoma cutaneum today. Are precisely established only фа...
Section: Articles about health
According to doctors, more than a half of men of 25-50 years suffer from frustration of the urinogenital sphere, but the minority sees a doctor from them. And in vain - even the insignificant discomfort in the field of generative organs can serve as a symptom of an illness fraught heavy посл...
Section: Articles about health
Each woman has preferences in the field of use of those goods which help us to look good, feel young and effective. Besides: selection process of favourite perfume, shampoo or decorative cosmetics already lightens the mood and serves as a peculiar stress medicine. Happens very offensively when the acquired perfumery and cosmetic products not only do not meet our expectations, but also becomes the reason of problems with health. Sources неприятн...
Section: Articles about health

