Leykostim
Application instruction:
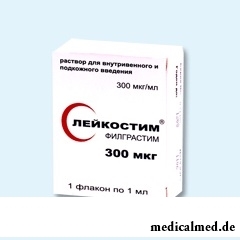 Leykostim – an immunomodulator.
Leykostim – an immunomodulator.
Form of release and structure
Dosage form – solution for intravenous and hypodermic administration: colourless or yellowish, transparent (150 mkg/ml – on 1 ml in bottles, 1 or 5 bottles in packaging planimetric cell, 1 packaging in a pack cardboard; 300 mkg/ml – on 1 or 1,6 ml in bottles, 1 or 5 bottles in packaging planimetric cell, 1 packaging in a pack cardboard; on 0,5 or 1 ml in syringes with pistons and the sealed needles, 1 or 5 syringes in a pack cardboard; 600 mkg/ml – on 0,8 ml in syringes with pistons and the sealed needles, 1 or 5 syringes in packaging planimetric cell, 1 packaging in a pack cardboard).
Active agent: filgrasty, in 1 ml of solution – 150 mkg (15 million ME), 300 mkg (30 million ME) or 600 mkg (60 million ME) (ME – the international units).
Auxiliary components: water for injections, acetic acid ice, acetate sodium trihydrate, polysorbate 80, a dextran 60 000, Mannitolum.
Indications to use
Leykostim apply:
- Treatment of a resistant neutropenia at patients with the developed stage of a human immunodeficiency virus (HIV infection with absolute number of neutrophils ≤1,0Õ109/l) for decrease in risk of development of bacterial infections;
- Therapy of a heavy chronic neutropenia for decrease in frequency and duration of infectious complications, and also increases in number of neutrophils;
- Mobilization of hemopoietic progenitors in peripheral blood at healthy donors for the subsequent their separation and allogenic transplantation;
- Mobilization of hemopoietic progenitors in peripheral blood for the subsequent their separation and transplantation after carrying out myelosuppressive chemotherapy;
- Reduction of duration of a neutropenia of the II-IV degree and decrease in frequency of a febrile neutropenia at patients with not myeloproliferative new growths after carrying out chemotherapy cytostatic drugs;
- Reduction of duration of a neutropenia and prevention of the complications caused by its development in the patients receiving miyeloablativny chemotherapy with the subsequent transplantation of marrow.
Contraindications
Absolute:
- Kostmann's syndrome (heavy inborn neutropenia) with cytogenetic disturbances;
- Neonatality period (till 28 in the afternoon lives);
- Pregnancy;
- Lactation (or feeding needs to be stopped);
- Use of high doses of cytotoxic himiopreparat (above the averages recommended);
- Hypersensitivity to drug.
With care filgrasty should apply at an acute myeloleukemia.
Route of administration and dosage
Leykostim enter subcutaneously (п / to) or intravenously (in/in). The way of introduction and a dose are defined by individually attending physician depending on a clinical situation. The hypodermic way of introduction is more preferable. In need of intravenous injection the drug is administered in a plastic container or a bottle from 5% dextrose solution. Solution of sodium of chloride of 0,9% is forbidden to be used!
It is impossible to dissolve drug to final concentration less than 2 mkg/ml.
Duration of intravenous infusion – 30 minutes.
The recommended doses:
- Treatment of a neutropenia after a course of cytotoxic chemotherapy: п / to or in/in on 5 mkg/kg of body weight of 1 times a day. For adequate assessment of efficiency of treatment it is recommended to count daily quantity of neutrophils in peripheral blood. It is necessary to continue therapy until the quantity of neutrophils does not exceed the expected minimum and will not reach the normal value exceeding 2,0x109/l. Duration of treatment can reach 12 days;
- Reduction of duration of a neutropenia and the prevention of the related complications after miyeloablativny chemotherapy with the subsequent bone marrow transplantation: п / to or in/in in a dose of 10 mkg/kg of body weight of the patient. The first dose is entered at least in 24 hours after chemotherapy, and at transplantation of marrow – not later than in 24 hours after infusion of marrow. After the maximum decrease in quantity of neutrophils, depending on dynamics of their number the daily dose is adjusted. If the maintenance of neutrophils in peripheral blood exceeds an indicator 1,0Õ109/l within 3 days in a row, Leykostim's dose is reduced twice. If the quantity of neutrophils decreases below a mark 1,0Õ109/l, the dose is increased to 10 mkg/kg again. If the absolute number of neutrophils exceeds 1,0Õ109/l within 3 days in a row, drug is cancelled;
- Mobilization of the hemopoietic stem cells: п / to in a daily dose of 5 mkg/kg of body weight (to patients after myelosuppressive chemotherapy) or in a dose of 10 mkg/kg (patients who did not study chemotherapy) daily within 5-7 days depending on the speed of increase in number of leukocytes in peripheral blood and effectiveness of separation. In 1 day prior to estimated date of the first separation (the 4th day of introduction of Leykostim) and further (up to day of the last separation) it is necessary to estimate quantity of neutrophils and leukocytes in peripheral blood. Tsitaferez carry out if the number of leukocytes reaches 5Õ109/l peripheral blood from the 5th day of administration of drug. After each separation in the sample intended for a cryopreservation count number of cells of CD34+ and yadrosoderzhashchy cells. Introduction of a filgrastim is stopped after achievement of the quantity of the cells cryopreserved by CD34+ sufficient for performing transplantation (not less than 2x106 on kg of mass of the patient). Safety and efficiency of use of drug for healthy donors are more senior than 60 years and 16 years are younger were not investigated;
- Heavy chronic neutropenia: п / to daily in a daily dose of 12 mkg/kg of body weight of the patient (at an inborn neutropenia) or 5 mkg/kg (at a periodic and idiopathic neutropenia). Treatment is continued until the quantity of neutrophils does steadily not exceed 1,5Õ109/l. Then select a minimal effective dose for maintenance of the achieved result. When carrying out a maintenance therapy Leykostim enter daily during the long period. 1-2 weeks later depending on reaction of the patient the daily dose can be increased or reduced twice. Further each 1-2 weeks adjust a dose for maintenance of quantity of neutrophils in the range from 1,5Õ109/l up to 10Õ109/l;
- The neutropenia associated with HIV infection: п / to daily 1 once a day. An initial dose – 1-4 mkg/kg of body weight, apply it before normalization of number of neutrophils (more than 2Õ109/l), usually 2 days for this purpose are required. In case of inefficiency of an initial dose it is increased to 5 mkg/kg/days. After achievement of therapeutic effect carry out the supporting treatment in a dose of 1-4 mkg/kg/days 2-3 times a week. Further for maintenance of quantity of neutrophils is normal (> 2Õ109/l) individual dose adjustment and prolonged treatment can be required.
At children with oncological diseases and a heavy neutropenia the profile of safety of Leykostim did not differ from that at adults. Therefore to children appoint the doses similar to doses of the adult patients receiving cytotoxic or myelosuppressive chemotherapy.
Correction of doses is not required to patients with a heavy liver or renal failure as their pharmakodinamichesky and pharmacokinetic indicators are similar to those at healthy volunteers.
Side effects
- Cardiovascular system: passing lowering of arterial pressure;
- Musculoskeletal system: muscle and/or bones pains (usually weak or moderate), an aggravation of the available pseudorheumatism;
- System of a hemopoiesis: anemia, thrombocytopenia;
- Nervous system: headache, increased fatigue;
- Respiratory system: infiltrates in lungs with development respiratory a distress syndrome of adults (is more often after the schemes of chemotherapy including Bleomycinum; their communication using a filgrastim is not clear);
- Alimentary system: diarrhea, hepatomegalia;
- Laboratory indicators: decrease in number of thrombocytes in peripheral blood, reversible increase in levels of a lactate dehydrogenase, alkaline phosphatase, uric acid, a glutamiltranspeptidaza in a blood plasma;
- Urinary system: dysuria (usually weak or moderate);
- Others: morbidity in the place of an injection; seldom (is more often later in/in introductions) – allergic reactions (about a half of them usually arises at introduction of the first dose), skin rash, a vasculitis, increase in a spleen, a rupture of a spleen, fibrinferments of vessels.
Special instructions
Treatment by Leykostim has to be carried out only under control of the oncologist or hematologist having experience of use of similar medicines. Before its appointment it is necessary to exclude such reasons of development of a passing neutropenia as viral infections.
Special attention needs to be paid to diagnosis of heavy chronic neutropenias to differentiate them from other hematologic diseases, such as a myeloleukemia, a myelodisplasia and aplastic anemia.
At a myelosis and a miyelodisplastichesky syndrome safety and efficiency of use of a filgrastim are not established. And with pretumor defeats of a myeloid sprout of a hemopoiesis Leykostim's appointment is not recommended to patients with these diseases since cells of some tumors can bear a receptor to a granulotsitarny colony stimulating factor. For this reason special attention should be paid to differential diagnosis between blast crisis of a myelosis and an acute myeloleukemia.
During therapy it is necessary to control constantly the spleen sizes (by a stomach palpation). According to data of researches, at a dose decline of a filgrastim increase in a spleen stops or, at least, is slowed down.
At a small number (about 3%) of the patients with Kostmann's Syndrome receiving filgrasty the leukosis and a miyelodisplastichesky syndrome – natural complications of this disease which connection using drug is not established were observed. In case of development of these complications Leykostim it is necessary to cancel.
In rare instances (less than 5%) at the patients who are receiving medical treatment filgrastimy the hyperleukocytosis was noted (increase in number of leukocytes higher than 100Õ109/l) therefore it is necessary to define quantity of leukocytes regularly. At their increase more than 50x109/l Leykosty it is necessary to cancel. If drug is used for mobilization of the hemopoietic stem cells, it needs to be cancelled at increase in number of leukocytes more than 70x109/l.
Approximately at 12% of patients with initially normal cytogenetics at a repeated research anomalies, including a monosomy 7 came to light. If cytogenetic disturbances are found in patients with a heavy inborn neutropenia, it is necessary to weigh advantages and possible risks of continuation of therapy. Each 12 months it is necessary to conduct cytogenetic and morphological researches of marrow.
It is important to consider what filgrasty does not prevent anemia and thrombocytopenia which often are a consequence of use of chemotherapeutic drugs in high doses. Therefore during treatment after chemotherapy it is regularly necessary to define quantity of thrombocytes and erythrocytes, and also hemoglobin level.
Considering the mechanism of pharmacological action of a filgrastim, its influence on the speed of psychomotor reactions and ability to concentration of attention is represented extremely improbable.
Medicinal interaction
Leykostim pharmaceutical is incompatible with solution of sodium of chloride of 0,9%.
Considering hypersensitivity of quickly sharing myeloid cells to cytotoxic himiopreparata, Leykostim in 24 hours prior to the beginning of a chemotherapeutic course and at least 24 hours after its termination is not recommended to use. Safety and efficiency of introduction of a filgrastim in one day with cytotoxic himiopreparata are not established. It is known that 5-ftoruratsit at simultaneous use with filgrastimy increases weight of a neutropenia.
At Leykostim's use for mobilization of the hemopoietic stem cells after carrying out chemotherapy it is necessary to consider that prolonged use of tsitostatik, such as карбоплатин, Melphalanum, кармустин (BCNU), can reduce efficiency of mobilization.
Terms and storage conditions
To store in the place, unavailable to children. To observe temperature condition 2-8 ºС.
Period of validity – 2 years.
Name of drug
Price
Drugstore
Except people, only one living being on the planet Earth – dogs suffers from prostatitis. Here really our most loyal friends.

Each person supports all life a SARS about 200 times. The peak of incidence falls on cold season, but to get sick from temperatures...
Section: Articles about health
Extracorporal fertilization – one of the most modern methods of controlling with infertility. So far he already helped a significant amount of married couples to become happy parents. Usually to the EKO procedure difficult and very expensive, resort in those...
Section: Articles about health
There is an opinion that at low temperatures safety of products is ensured longer and better thanks to what the refrigerator is considered the most suitable place for storage of food. In most cases it is fair, however there is a number of products for which low temperatures – the main reason of their premature damage. Storage in the refrigerator leads to their bystry rotting, emergence of a mold, is followed by loss of vitamins and tastes. What products it is better to remove...
Section: Articles about health
Each person knows that fervescence is an illness sign. However about existence of diseases can to suite...
Section: Articles about health
The naturopathy sometimes moves as the new direction of medicine, something like fashionable hobby, and there is nothing farther from the truth. This most ancient direction, the word "naturopathy" is translated as "treatment by the nature", and, no doubt, treatment приро...
Section: Articles about health
Within several decades of our compatriots convinced that the use of butter nasty affects a condition of coronary vessels. As a result the reputation of a product was impaired thoroughly a little, and many almost ceased to include it in the diet, having given preference "to safer" to vegetable fats. Meanwhile, the last researches showed that harm of butter for health is strongly exaggerated. But the product has a number of unique properties, to...
Section: Articles about health
Proofs of efficiency of Mildronate at treatment of coronary heart disease with stenocardia can be found in many publications to...
Section: Articles about health
The hysteromyoma is diagnosed more than at a third of women 35 years are more senior. This high-quality new growth which at early stages successfully resolves by means of medicines. It is necessary to resort to an operative measure only in those a case...
Section: Articles about health
About 20% of the population of our planet have a hypertension (permanent increase in arterial pressure). This disease has an adverse effect on the standard of living, reduces working capacity, and in the absence of systematic treatment threatens with such complications as a myocardial infarction, a stroke and other heavy illnesses which can result in disability or sudden death. Most of patients for maintenance of pressure at more or less acceptable level accept appointed doctors лекарст...
Section: Articles about health
The depression not without reason is considered one their main troubles of our century: for scientific and technical progress, acceleration of rate of life and a surplus...
Section: Articles about health
All know that self-treatment is dangerous. However absolutely it is almost impossible to do without it. Rate of modern life does not allow to handle each small trouble to the doctor and information on ways of independent rendering medical the help...
Section: Articles about health
Aspirin (acetylsalicylic acid) – one of those drugs which are known literally to all. It is available in each home first-aid kit, and many accept it at the first signs of an indisposition, often without having a fair idea of properties and therapeutic effect of drug. Meanwhile, impact of aspirin on a human body is very various, and is not always favorable. About it it is important to foreknow, in order to avoid emergence of problems with health....
Section: Articles about health
The majority of gynecologic diseases prove three main signs, each of which speaks about need of a visit to the gynecologist. Certainly, it is possible to establish the exact diagnosis only after inspection, but on the basis of some signs it is possible to assume existence of this or that pathology. Let's consider symptoms of the female diseases which are found most often....
Section: Articles about health
Many of us, probably, noticed more than once that from intellectual loadings at some point the brain as though "overheats" also "assimilation"...
Section: Articles about health
All like to sing. Small children with pleasure are engaged in a vocal, not especially thinking of hit in a melody. Adults most often hesitate, being afraid to show lack of talents in this area, and it is vain: singing is very useful for health....
Section: Articles about health
All are familiar with cold, and practically everyone believes that he has sufficient knowledge and experience that correctly to treat it. In practice most of people makes mistakes in attempts to get rid of rhinitis, and divides numerous delusions it....
Section: Articles about health
Contrary to popular belief, the multiple sclerosis (MS) is not connected neither with sclerous changes of walls of vessels, nor about age...
Section: Articles about health
The healthy nutrition is the invariable principle of health and good health for long years of the woman. Nevertheless, in a diet at each stage of life there are the features allowing to support an organism by those substances which are most necessary...
Section: Articles about health
Any of us is not insured from a heavy illness of the loved one. Happens and so that someone from family members becomes the bed patient, and remains in such state for a long time. It extremely suppresses both the most injured, and all its house which life considerably changes....
Section: Articles about health
Weakness of an ankle joint – very widespread problem. Its existence is demonstrated by tendency to a podvorachivaniye of legs п...
Section: Articles about health
For the time being the perspective of heart diseases seems to most of people remote and foggy. But sooner or later practically each adult faces extremely unpleasant feelings: sudden stethalgia. To be consoled at this time in a thought of t...
Section: Articles about health
For anybody not a secret that our country is one of the most "drinking" in the world. At clear understanding that the use of hard alcoholic drinks – occupation extremely harmful, most of Russians belong to alcoholism with unjustified loyalty. Apparently, existence of a set of myths in which tendency to excessive libations looks nearly positively is explained by it. It is worth getting acquainted with the most widespread of similar delusions and to be convinced in them not...
Section: Articles about health
Sugar - the digestible refined product which is not of special value for an organism of the modern person. Use...
Section: Articles about health
Nightmares belong to the most unpleasant frustration. Statistically, they happen at 4% of adults, and almost at 70% of children and teenagers. During a nightmare of people dreams himself in extremely difficult, life-threatening situation. It wakens suddenly, in...
Section: Articles about health
The modern person not always manages to find housing in the environmentally friendly region and such work which would not do harm to health. With food stuffs at first sight the situation is much better: shops are overflowed with goods which are positioned by producers as very useful and absolutely safe. Many Russians are absolutely sure that the choice of products with marking "bio", "эко" or "organik" guarantees them and members of their families an optimal variant of food. To a sozhala...
Section: Articles about health