





Movalis
Application instruction:
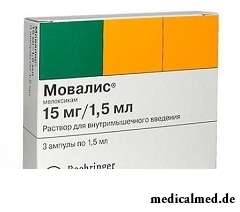 Movalis – the medicine with antiinflammatory, analgeziruyushchy and febrifugal action applied at a symptomatic treatment of a pseudorheumatism and an osteoarthritis.
Movalis – the medicine with antiinflammatory, analgeziruyushchy and febrifugal action applied at a symptomatic treatment of a pseudorheumatism and an osteoarthritis.
Form of release and structure
Movalis is issued in the following dosage forms:
- Tablets: from pale yellow till yellow color, on the one hand – bent risk and a code, with another (convex with slanted edge) – a logo of the producer, the surface roughness is allowed (in blisters on 10 pieces, on 1 or 2 blisters in a cardboard pack);
- Suspension for intake: viscous, yellowish with a green shade (in dark glass bottles on 100 ml, on 1 bottle in a cardboard pack complete with a spoon dosing);
- Solution for intramuscular introduction: transparent, yellow color with a green shade (in colourless glass ampoules on 1,5 ml, on 3 or 5 ampoules in blister strip packagings or pallets, on 1 or 2 packagings or the pallet in a cardboard pack);
- Suppositories rectal: yellowish-green, smooth, in the basis – deepening (in blister strip packagings on 6 pieces, on 1 or 2 packagings in a cardboard pack).
Is a part of 1 tablet:
- Active ingredient: to meloksika – 7,5 or 15 mg;
- Auxiliary components (7,5mg/15 mg): magnesium stearate – 1,7/1,7 mg, K25 povidone – 10,5/9 mg, monohydrate of lactose – 23,5/20 mg, a sodium citrate dihydrate – 15/30 mg, кросповидон – 16,3/14 mg, microcrystallic cellulose – 102/87,3 mg, colloid silicon dioxide – 3,5/3 mg.
For intake is a part of 5 ml of suspension:
- Active ingredient: to meloksika – 7,5 mg;
- Auxiliary components: crimson fragrance – 10 mg, sodium benzoate – 7,5 mg, 70% sorbitol – 1750 mg, monohydrate of citric acid – 6 mg, sodium saccharinate – 0,5 mg, a gietelloza – 5 mg, a dihydrosodium phosphate dihydrate – 100 mg, ксилитол – 750 mg, 85% глицерол – 750 mg, colloid silicon dioxide – 50 mg, the purified water – 2463,5 mg.
For intramuscular injections is a part of 1 ml of solution:
- Active ingredient: to meloksika – 10 mg;
- Auxiliary components: glycine – 7,5 mg, Megluminum – 9,375 mg, sodium chloride – 4,5 mg, sodium hydroxide – 0,228 mg, half-oxameasures of 188 - 75 mg, glycofurfural – 150 mg, water for injections – 1279,482 mg.
Rectal is a part of 1 suppository:
- Active ingredient: to meloksika – 7,5 or 15 mg;
- Auxiliary components: суппоцир BP (suppozitorny weight), глицерилгидроксистеарат polyethyleneglycol (глицерилгидроксистеарат macrogoal).
Indications to use
Movalis appoint for symptomatic therapy of the following diseases:
- Pseudorheumatism;
- Osteoarthritis, including degenerative diseases of joints, arthrosis;
- Ankylosing spondylitis.
Contraindications
Absolute:
- Combination of bronchial asthma (full or partial), recuring polypose of okolonosovy bosoms and a nose with intolerance of acetylsalicylic acid or other non-steroidal anti-inflammatory drugs (now or instructions in the anamnesis);
- Peptic ulcer and/or perforation of a stomach and duodenum (at an aggravation or recently postponed);
- Active gastrointestinal bleedings; the postponed recently cerebrovascular bleedings or the confirmed diseases of coagulant system of blood;
- Disease Krone or ulcer colitis (at an aggravation);
- The progressing diseases of kidneys, heavy renal failure (at the confirmed hyperpotassemia; at clearance of creatinine it is less than 30 ml in a minute; in cases if the hemodialysis is not carried out);
- Liver failure in a severe form;
- The uncontrollable expressed heart failure;
- The postoperative pains connected with performing shunting of coronary arteries;
- Rare hereditary intolerance of a galactose (at purpose of drug in the form of tablets (47/20 mg of lactose respectively are a part of the maximum daily dose of Movalis of 7,5/15 mg));
- Rare hereditary intolerance of fructose (at purpose of drug in the form of suspension for intake (2450 mg of sorbitol are a part of the maximum daily dose of drug));
- Age up to 18 years (at purpose of drug in the form of injection solution); up to 12 years (at purpose of drug in the form of tablets, suspension for intake, suppositories, except for Movalis's use at treatment of a juvenile pseudorheumatism);
- Pregnancy and period of breastfeeding;
- Hypersensitivity to drug components, and also to acetylsalicylic acid and other non-steroidal anti-inflammatory drugs (there is a probability of development of cross hypersensitivity).
Relative (Movalis it is necessary to apply with care at the following diseases / states):
- Diseases of peripheral arteries;
- Congestive heart failure;
- Digestive tract diseases in the anamnesis (at an infection Helikobakter of a pilora);
- Coronary heart disease;
- Cerebrovascular diseases;
- Renal failure (at clearance of creatinine from 30 to 60 ml a minute);
- Diabetes mellitus;
- Lipidemia and/or dislipidemiya;
- Frequent alcohol intake and smoking;
- Long therapy by non-steroidal anti-inflammatory drugs;
- Co-administration with a methotrexate in a dose from 15 mg a week;
- The combined use with selective serotonin reuptake inhibitors, antiagregant, anticoagulants, peroral glucocorticosteroids;
- Advanced age.
Route of administration and dosage
Movalis is recommended to apply shortly in the smallest effective dose as it reduces probability of development of side effects.
Tablets and suspension for intake:
Orally it is more preferable to accept drug to food.
As a rule, appoint the following mode of dosing (daily dose):
- Osteoarthrosis – 7,5 mg (increase in a dose is possible twice);
- The pseudorheumatism ankylosing a spondylitis – 15 mg (reduction of a dose is possible twice).
At the increased risk of development of side effects treatment is recommended to begin with a dose 7,5 mg a day.
Frequency rate of use – once a day.
To children up to 12 years at therapy of a juvenile pseudorheumatism appoint Movalis in the form of suspension for intake. The dose is calculated on the basis of body weight – 0,125 mg/kg (as much as possible – 7,5 mg a day). It is recommended to apply the following mode of dosing (amount of active substance/volume of suspension):
- 12 kg: 1,5 mg / 1 ml;
- 24 kg: 3 mg / 2 ml;
- 36 kg: 4,5 mg / 3 ml;
- 48 kg: 6 mg / 4 ml;
- From 60 kg: 7,5 mg / 5 ml.
The maximum dose at children of 12-18 years with a juvenile pseudorheumatism – 0,25 mg/kg, but no more than 15 mg a day.
Solution for intramuscular introduction:
Intramuscular introduction of Movalis is usually appointed only during the first 2-3 days of therapy then pass to use of enteral forms of drug.
The recommended daily dose makes 7,5 mg or 15 mg (as much as possible), frequency rate of use – once a day. The dose is defined by weight of course of inflammatory process and intensity of pains.
Injection solution needs to be entered deeply intramusculary (intravenous use is contraindicated). It is not necessary to mix Movalis with other medicines in one syringe.
Suppositories rectal:
Movalis is recommended to apply 7,5 mg in a daily dose, according to indications its increase up to 15 mg is possible.
The patient with an end-stage of a renal failure who is on a hemodialysis, Movalis in any dosage form appoint in a dose no more than 7,5 mg a day. Correction of the mode of dosing at moderate or insignificant functional disturbances of kidneys (at clearance of creatinine from 30 ml a minute) is not required.
At simultaneous use of various dosage forms of drug the total daily dose of Movalis should not exceed 15 mg a day.
Side effects
- Respiratory system: seldom – bronchial asthma (at patients with an allergy to acetylsalicylic acid or other non-steroidal anti-inflammatory drugs);
- Alimentary system: often – pains in a stomach, dyspepsia, diarrhea, vomiting, nausea; infrequently – the gastrointestinal bleedings (proceeding it is explicit or hidden), abdominal distention, gastritis, a lock, an eructation, stomatitis; seldom – an esophagitis, gastroduodenal ulcers, colitis; very seldom – perforation of digestive tract;
- Nervous system: often – a headache; infrequently – drowsiness, dizziness;
- Cardiovascular system: infrequently – increase in arterial pressure, feeling of "inflows" of blood to the person; seldom – heartbeat;
- Urinary system: infrequently – changes of functional indicators of kidneys (increase in blood serum of level of urea and/or creatinine), disturbances of an urination, including an acute ischuria; very seldom – an acute renal failure;
- Hemopoietic system: infrequently – anemia; seldom – thrombocytopenia, a leukopenia, change of quantity of blood cells, including changes of a leukocytic formula;
- Immune system: infrequently – hypersensitivity reactions of immediate type; with an unknown frequency – anaphylactoid and/or anaphylactic reactions, an acute anaphylaxis;
- Mentality: seldom – changeability of mood; with an unknown frequency – confusion of consciousness, a disorientation;
- Sense bodys: infrequently – вертиго; seldom – conjunctivitis, a sonitus, vision disorders, including a sight illegibility;
- Hypodermic fabrics and skin: infrequently – a Quincke's disease, an itch, skin rash; seldom – a small tortoiseshell, Stephens-Johnson's syndrome, a toxic epidermal necrolysis; very seldom – violent dermatitis, a mnogoformny erythema; with an unknown frequency – a photosensitization;
- Biliary tract and liver: infrequently – tranzitorny changes of indicators of function of a liver (in particular, increase in bilirubin or activity of transaminases); very seldom – hepatitis;
- The general frustration and reactions in an injection site of injection solution: often – hypostasis and pain in an injection site; infrequently – hypostases.
At combined use of Movalis with the drugs oppressing marrow (for example, with a methotrexate), the cytopenia can develop.
The gastrointestinal bleeding, perforation or ulcer connected with performing therapy can lead to a lethal outcome.
As well as at use of other non-steroidal anti-inflammatory drugs, during treatment by Movalis there is a probability of development of a nephrotic syndrome, glomerulonephritis, renal medullary necrosis and intersticial nephrite.
Special instructions
At Movalis's use from skin such essential disturbances as Stephens-Johnson's syndrome, a toxic epidermal necrolysis and exfoliative dermatitis can develop. Special attention needs to be paid to patients with the undesirable phenomena from mucous membranes and skin, and also reactions of hypersensitivity to effect of drug, in particular, if similar reactions were observed when carrying out the previous courses of treatment. In most cases disturbances from skin develop for the first 30 days of use of drug. Such side effects can sometimes become the reason of cancellation of Movalis.
During treatment bleeding, perforation and ulcers of digestive tract can arise at patients with existence or lack of alarming symptoms or data on digestive tract diseases in the anamnesis. For elderly patients of an effect of these complications are more serious.
Patients with gastrointestinal diseases need to undergo regular control. At development of gastrointestinal bleedings or cankers of digestive tract Movalis's use should be interrupted.
Treatment by drug can lead to increase in risk of development of cardiovascular thromboses, attacks of stenocardia, myocardial infarction (sometimes – with a lethal outcome). The risk of emergence of such disturbances increases at long therapy, and also at patients with the above-stated diseases in the anamnesis and in cases of predisposition to their emergence.
Performing treatment by Movalis at patients with the reduced volume of the circulating blood or with the lowered renal blood-groove can become the reason of development of a decompensation of a skrytoprotekayushchy renal failure as drug inhibits synthesis of prostaglandins participating in maintenance of renal perfusion in kidneys. As a rule, after Movalis's cancellation functional disturbances of kidneys take place. Most elderly patients are subject to risk of development of these reactions; patients with congestive heart failure, dehydration, cirrhosis, acute functional disorders of kidneys or a nephrotic syndrome; patients after carrying out serious surgical interventions which can lead to emergence of a hypovolemia. At such patients at the beginning of therapy it is necessary to control function of kidneys and a diuresis carefully. Also the probability of development of the renal failure proceeding in the latent form increases at simultaneous use with antagonists of receptors of angiotensin II, diuretic medicines, inhibitors of an angiotensin-converting enzyme.
At simultaneous use of Movalis with diuretics the delay of sodium, potassium and water can develop, and also decrease in a natriuretic effect of diuretic drugs is possible. Because of it at predisposed patients symptoms of heart failure or hypertensia can amplify (it is necessary to carry out adequate hydration and to exercise careful control of a condition of such patients).
Periodically during therapy increase in activity of transaminases in blood serum or other functional indicators of a liver is possible. This increase in most cases was insignificant and passing. If similar disturbances have essential character, or their expressiveness does not decrease over time, it is necessary to interrupt treatment and further to make observation of the revealed laboratory changes.
Before Movalis's appointment, and also when performing the combined treatment it is necessary to conduct a research of a functional condition of kidneys.
The exhausted or weakened patients need careful control of their state as can worse transfer the side effects caused by therapy.
It is necessary to consider that Movalis can mask symptoms of the main infectious disease.
Drug can exert impact on fertility therefore Movalis's use is not recommended to the women having difficulties with conception.
When performing potentially dangerous types of the works demanding bystry psychomotor reactions and the increased concentration of attention (including control of motor transport) it is necessary to consider a possibility of development of vision disorders, dizzinesses, drowsiness or other disturbances from the central nervous system.
Medicinal interaction
At combined use of Movalis with some drugs there can be following effects:
- Selective serotonin reuptake inhibitors: the risk of emergence of gastrointestinal bleedings increases;
- Other inhibitors of synthesis of prostaglandins, including salicylates and glucocorticoids: the risk of developing of gastrointestinal bleedings and formation of ulcers in digestive tract increases (it is caused by a synergism of effect of medicines; the combination of drugs is not recommended);
- Anti-hypertensive drugs (diuretics, beta adrenoblockers, vazodilatator, inhibitors of an angiotensin-converting enzyme): their efficiency decreases;
- Methotrexate: tubular secretion decreases and its concentration in plasma without change of pharmacokinetics and hematologic toxicity increases (simultaneous use with doses more than 15 mg of a methotrexate a week is not recommended; it is necessary to control constantly function of kidneys and number of blood cells);
- Antagonists of receptors of angiotensin II: decrease in glomerular filtering that can lead to development of an acute renal failure, in particular against the background of functional disturbances of kidneys (at purpose of a combination of these drugs it is necessary to control function of kidneys) amplifies;
- Cyclosporine: its nephrotoxicity amplifies;
- Lithium drugs: concentration of lithium in plasma (during Movalis's appointment, change of doses of drugs of lithium or at their cancellation it is necessary to carry out monitoring of concentration of lithium) increases;
- Diuretics: the risk of development of an acute renal failure at dehydration increases;
- Holestiramin: speed of removal of a meloksikam increases;
- Intrauterine contraceptive drugs: their efficiency decreases.
Also at purpose of the combined treatment it is necessary to consider the following cautions:
- Other non-steroidal anti-inflammatory drugs: combined use is not recommended;
- Peroral hypoglycemic medicines: it is necessary to consider a possibility of development of interaction;
- Diuretics: it is necessary to carry out adequate hydration, prior to therapy it is necessary to conduct a research of function of kidneys;
- Medicines with the known ability to inhibit CYP2C9 and/or CYP3A4: it is necessary to consider a possibility of pharmacokinetic interaction.
Terms and storage conditions
To store in the place, unavailable to children.
Period of validity:
- Tablets and suspension for intake: 3 years at a temperature up to 25 °C;
- Solution for intramusculary introduction: 5 years in the place protected from light at a temperature up to 30 °C;
- Suppositories rectal: 3 years at a temperature up to 30 °C.
Movalis period of storage in the form of suspension after opening of a bottle – 30 days.
Name of drug
Price
Drugstore
Movalis тбл 15 mg No. 10, Boehringer Ingelheim
548 rub.
 Network of the Moscow drugstores of IFC
Network of the Moscow drugstores of IFCMovalis сусп. for внутр. comment 7,5mg/5ml фл. 100 ml, Boehringer Ingelheim
644 rub.
 Network of the Moscow drugstores of IFC
Network of the Moscow drugstores of IFCMovalis solution for инъ 15 mg 1.5ml amp No. 3, Boehringer Ingelheim
648 rub.
 Network of the Moscow drugstores of IFC
Network of the Moscow drugstores of IFCMovalis тбл 7,5mg No. 20, Boehringer Ingelheim
671 rub.
 Network of the Moscow drugstores of IFC
Network of the Moscow drugstores of IFCMany drugs initially moved ahead in the market as drugs. Heroin, for example, was initially brought to the market as children's cough medicine. And cocaine was recommended by doctors as anesthesia and as the means increasing endurance.

Doctors claim that the people not so familiar with a dorsodynia occur among adult Russians very seldom. At the same time подавляющ...
Section: Articles about health
All know that self-treatment is dangerous. However absolutely it is almost impossible to do without it. Rate of modern life does not allow to handle each small trouble to the doctor and information on ways of independent rendering medical the help...
Section: Articles about health
Not everyone can brag of the shining Hollywood smile. Even the person who is regularly visiting the stomatologist and watching of oral cavities over health periodically has problems: enamel of teeth darkens under the influence of some products, on it the deposits giving to teeth a grayish or yellowish shade collect....
Section: Articles about health
The problem of diagnosis was and remains to one of the most important in medicine. From that, the reason недо will be how precisely defined...
Section: Articles about health
Popular joke that there are no healthy people, and is nedoobsledovanny, most of us considers an honest truth, continually it is necessary to hear that all of us are sick hardly from a school bench. It is hard to say, whether so it actually because...
Section: Articles about health
Food with the increased content of sugar is attractive to most of people - it is scientifically confirmed fact. Business here not in intemperance or dissoluteness: the sweet food is associated since childhood with feeling of rest and safety which tests the kid when it absorbs maternal milk. Besides, getting into a human body, sugar strengthens production of "happiness hormones" which all of us so need. And still life of sweet teeth seldom happens cloudless: their too big loss...
Section: Articles about health
Helminthosis is one of the most widespread diseases. Statistically, with any species of helminths it is infected porridges...
Section: Articles about health
The Genetically Modified Organisms (GMO) are plants or animals (as a rule, agricultural) to whose genotype purposeful changes were made. Opposition of supporters and opponents of inclusion of such organisms in foodstuff всег...
Section: Articles about health
According to World Health Organization, every third inhabitant of Earth has excess weight, and every tenth has obesity. The reason of this phenomenon, according to specialists, roots in one not very comforting fact: most of people consume much more calories, than it is necessary. How it turns out what we overeat? Why it is so difficult to refuse an excess portion tasty or additives? Let's try to find out what factors prevent us to eat food with reasonable moderation....
Section: Articles about health
Life expectancy in various regions of Earth is not identical. Exert impact on it social stability, economic бл...
Section: Articles about health
Iodine - one of thirty most important microelements in our organism. The main role of iodine consists in synthesis of thyroid hormones of a thyroid gland - the substances which are responsible for the majority of exchange processes of an organism. It is known that thyroid hormones consist...
Section: Articles about health
History of mankind contains several tens of epidemics whose emergence was compared by eyewitnesses and historians to doomsday. The most terrible of them claimed the lives of millions of people, having made even the whole people to the person of the earth. What they − the diseases striking terror? Whether it managed to the person to find treatment, or he is still powerless before forces of nature?...
Section: Articles about health
Partial and the more so full loss of hearing significantly reduces quality of life. Difficulties with communication lead to loneliness and замкн...
Section: Articles about health
Cold is such painful that each sigh becomes a victory, heat "knocks" down, and the ache in joints forces to think only of pain. Some people with approach of the first symptoms of cold make the self-sacrificing decision to have a disease standing, and to a beam...
Section: Articles about health
Color of plants is caused by presence at them of certain chemical compounds. Let's talk about what is meant by various colors of vegetables and fruit and what properties they give them....
Section: Articles about health
Antibiotics - - it is possible to call the chemical compounds suppressing growth of bacteria the break in the field of medicine which allowed to save persons...
Section: Articles about health
Striya (extension) are the defects of skin having an appearance of direct or wavy strips from 1 to 10 cm long and 1-5 mm wide. In most cases at women of a striya are located on a stomach, hips, a breast or buttocks. At athletes they can appear on shoulders and внутренн...
Section: Articles about health
The nature does not stand stagnation and monotony. It is known that tissues of a human body atrophy if do not receive necessary loadings. It fully belongs also to a cerebral cortex: when it is not given full-time job, it begins to function worse. As a result memory decreases, the person becomes less bright, acquires information more slowly, hardly switches from one thought to another. There are problems at work, difficulties with communication and career development. These it is unpleasant...
Section: Articles about health
The cosmetics intended for improvement of a condition of skin, nails and hair are used by each woman. Expenses on регуля...
Section: Articles about health
Statistically, pathologies of a thyroid gland in the world more than 500 million people have. Failures in work of this body lead to heavy disbolism, development of heart diseases, vessels, a reproductive and nervous system. In hard cases excessive...
Section: Articles about health
Any person who faced a disease knows that treatment costs expensive. It belongs also to consultations of qualified specialists, and to the diagnostic procedures which are not included in the list of obligatory medical services. The question of cost of medicines is not so unambiguous: almost each drug is produced several producers at once, and the price of medicine can differ many times. In such situation there is a sense to understand in what differ from each other original environments...
Section: Articles about health
It is possible to find the extensive range of fruit and vegetables in modern shops. Russians already got used that on counters in any...
Section: Articles about health
Work of a brain is extremely complex and in many respects is not studied yet. It is confirmed also by the features of thought processes which are shown when the person sleeps. Let's tell about some of them....
Section: Articles about health
For most of the working people the problem of having a snack is particularly acute enough. Sooner or later there is a question: what can be eaten quickly between a breakfast and a lunch or a lunch and leaving from service so that to receive necessary power feed, but not to overload an organism with harmful components or excess calories? We bring to your attention the list of products which quite conform to these requirements....
Section: Articles about health
The main role in development of a peptic ulcer of a stomach and duodenum the bacterium Helikobakter plays pilor. Activity and Wuxi...
Section: Articles about health
Summer in the heat. Many are going to spend vacation abroad. Travelers the tender seas, rest on beaches wait, for sightseeing, campaigns on natural and cultural reserves. But, unfortunately, on vacation also problems about health can wait for us...
Section: Articles about health
Residents of big cities quite often have a disease which is known as the syndrome of chronic fatigue (SCF) today. This illness affects the people belonging to various social and demographic groups and living on all continents. Most of all SHU are subject women aged from 25 up to 45 years. Statistically, the number of cases fluctuates in the different countries from 10 to 37 people on 100 thousand, but specialists believe that these figures are significantly underestimated as people, страдающ...
Section: Articles about health

