Antithrombin III human
Application instruction:
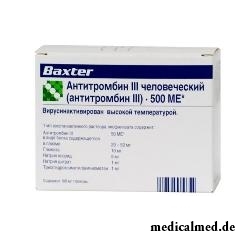 Antithrombin III human – antithrombin III drug, anticoagulant of direct action.
Antithrombin III human – antithrombin III drug, anticoagulant of direct action.
Form of release and structure
Dosage form – lyophilisate for preparation of solution for infusions: the friable firm mass or powder of pale green or pale yellow color (on 500 international units (IU) in bottles, in a cardboard box 1 bottle and a set for dissolution and introduction: 1 bottles of solvent (10 ml), needle filter, one-time needle, needle adapter, needle - "butterfly", airwater needle; on 1000 ME in bottles, in a cardboard box 1 bottle and a set for dissolution and introduction: 1 bottles of solvent (20 ml), needle filter, one-time needle, needle adapter, needle - "butterfly", airwater needle).
Active agent of Antithrombin III human – antithrombin III, in 1 ml of ready solution – 50 ME (1 ME solutions it is equivalent to activity of antithrombin III in 1 ml of normal fresh plasma of the person), crude protein – 20-50 mg.
Auxiliary components: Tris (hydroxymethyl) aminomethane, sodium chloride, Dextrosums monohydrate, citrate sodium dihydrate.
Solvent: water for injections.
Indications to use
Use of Antithrombin III human is shown to patients with the inborn or acquired insufficiency of antithrombin III in a blood plasma. The acquired insufficiency of antithrombin can be a consequence of various clinical disturbances, including the increased consumption or loss of protein, antithrombin synthesis disturbance.
Drug is appointed for prevention of trombotichesky and tromboembolic episodes by the patient with activity of antithrombin in plasma by less than 70% of the established norm.
Indications for introduction:
- surgeries at patients with inborn insufficiency of antithrombin III;
- the period pregnancy and childbirth at patients with inborn insufficiency of antithrombin III;
- thrombosis at patients with inflammatory diseases of a bladder or a nephrotic syndrome, or risk of its development;
- syndrome of the disseminated intravascular blood coagulation or risk of its development at the septic complications, the combined injury, a preeclampsia, shock and other states connected with an acute consumption coagulopathy;
- surgical intervention or bleeding at a heavy liver failure, especially at the patients who are on therapy by concentrates of factors of coagulation;
- absence or insufficient answer to heparin.
Contraindications
- the instruction in the anamnesis on heparin - the induced thrombocytopenia;
- age up to 6 years;
- hypersensitivity to drug components.
During pregnancy and breastfeeding human it is possible to appoint Antithrombin III only according to vital indications.
Route of administration and dosage
Lyophilisate is intended for intravenous (in/in) drop introductions.
Infusion solution is prepared before direct introduction, using the set enclosed for dissolution and introduction and strictly following rules of an asepsis and antiseptics. Heating still the closed bottle with solvent up to the temperature is not above 37 °C, having exempted bottles with lyophilisate and solvent from protective covers, rubber bungs on each of bottles should be disinfected. Having exempted since one end from a protective cap a needle adapter, to pierce a needle a bottle stopper with solvent. Without touching a needle, to remove a protective cap from other its end. Then, having turned a bottle with solvent, the free end of a needle it is necessary to pierce a rubber bung of a bottle with lyophilisate and under the influence of vacuum solvent will come to a bottle with lyophilisate. After removal of a needle from a bottle with drug easy rotation or rocking it is necessary to accelerate lyophilisate dissolution. For sedimentation of the formed foam after full dissolution of drug the stopper of a bottle is pierced an airwater needle and then deleted. The intake of the prepared solution is made by the one-time sterile syringe with the put-on needle filter. Having visually investigated solution and having convinced of lack of a turbidity or foreign inclusions, it is necessary to change a needle filter for a needle - "butterfly". Then it is necessary slowly in/in to enter solution, without exceeding the most admissible rate of administering – 5 ml a minute. Unused solution needs to be utilized according to the established rules.
The doctor with experience of therapy of insufficiency of antithrombin has to carry out purpose of drug and treatment.
The dose at inborn insufficiency is chosen for each patient individually, taking into account the family anamnesis of rather thromboembolic states, results of laboratory researches and the existing clinical risk factors.
Initial dose at inborn insufficiency usually appoint from calculation on 30-50 ME to 1 kg of weight. Further the single dose, frequency rate of introduction and duration of treatment are adapted to indicators of biochemical researches and a clinical condition of the patient.
The dose, frequency of introduction and the period of replacement therapy for each patient with the acquired insufficiency are defined on the basis of biochemical indicators of level of antithrombin in plasma, considering the diagnosis of a basic disease, the available signs of the increased metabolism of antithrombin and severity of a clinical state.
Proceeding from empirical data, when calculating a necessary dose use compliance in which for increase in activity of antithrombin in plasma of the person approximately for 2% it is necessary to enter on 1 ME antithrombins on 1 kg of body weight.
For definition of an initial dose of drug (ME) it is necessary to increase the nominal body weight of the patient by a difference between the target and initial objective of activity of antithrombin (which are expressed as a percentage) and to increase on 0,5.
Initial target activity of antithrombin has to be the established pathology, sufficient for achievement of target activity of antithrombin at replacement therapy. For maintenance of effective level it is necessary to take laboratory measurements of activity of antithrombin not less than 2 times a day, it is desirable – just before the following administration of drug. At the increased antithrombin metabolism the dose should be adjusted.
Activity of antithrombin throughout a course of therapy should be maintained at the level of higher than 80% (normal activity of antithrombin at adults – 80-120%) in the absence of need for maintenance of other effective level connected with clinical features.
Side effects
- cardiovascular systems: lowering of arterial pressure (ABP), tachycardia, reddening of integuments;
- alimentary system: nausea, vomiting;
- immune system: skin rash, allergic reactions (including hypersensitivity), a Quincke's disease, a heavy anaphylaxis (including shock), a generalized small tortoiseshell;
- nervous system: concern, a headache, feeling of a pricking in a body;
- respiratory system: goose breathing;
- laboratory and tool indicators: decrease in maintenance of thrombocytes twice or quantity of thrombocytes less than 100 000/mkl;
- general reactions: drowsiness, a fever, constraint in breasts, heparin - the induced thrombocytopenia with antibodies (type II), fever;
- local reactions: burning and a pricking in an injection site.
Special instructions
Because of risk of development of allergic reactions and hypersensitivity during the entire period of infusion it is necessary to watch a condition of the patient carefully. Prior to treatment of patients it is necessary to inform on possible development of early symptoms of reactions of hypersensitivity which a generalized small tortoiseshell, decrease in the ABP, feeling of constraint in a breast, goose breathing, an anaphylaxis, and about need of the immediate message to the doctor in case of their emergence treats.
Use of drug has to be carried out in the presence of means of antishock therapy.
At in administration of the drug made of a blood plasma or blood of the person infection with the casing-free virus, such as B19 parvovirus constituting serious danger to patients with an immunodeficiency or increased by an erythrogenesis (including hemolitic anemia) and pregnant women (risk of infection of a fruit) is possible.
The patient should be vaccinated against the hepatitises A and B at regular or repeated purpose of the drugs of antithrombin received from plasma of the person.
The specific patient needs carrying out registration of number of a series and the name of each entered Antithrombin III human.
From first minutes after the beginning of administration of antithrombin at combined use with heparin it is necessary to control often and regularly extent of anti-coagulation for the purpose of dose adjustment of heparin and prevention of excess decrease in coagulability of blood. Also because of the existing risk of decrease in level of antithrombin against the background of prolonged use of unfractionated heparin daily measurement of level of antithrombin and correction of an individual dose if necessary is required.
Antithrombin does not influence ability of the patient to control of vehicles and mechanisms.
Medicinal interaction
At simultaneous use of replacement therapy by antithrombin with heparin in therapeutic doses:
- the risk of bleeding increases;
- effect of antithrombin amplifies;
- metabolism of antithrombin accelerates, causing essential decrease in T1/2 of antithrombin.
Therefore at the increased risk of bleeding combined use of heparin and antithrombin should be accompanied with careful control of a clinical condition of the patient and his biochemical data.
It is impossible to mix Antithrombin III human with other medicines.
Terms and storage conditions
To protect from children.
Not to freeze, store at a temperature of 2-8 °C.
Period of validity – 3 years.
Name of drug
Price
Drugstore
Antithrombin III a human bottle Complete with solvent and set for dissolution and introduction (a one-time needle, a needle filter, a needle adapter, an airwater needle, a needle - 500 ml
11000 rub.
 Apteka вер.ру, LLC
Apteka вер.ру, LLCAntithrombin III human lyophilisate + solvent + set 1000ME 1 of piece
17500 rub.
 Apteka вер.ру, LLC
Apteka вер.ру, LLCAntidepressant Klomipramin causes an orgasm in 5% of patients.

Memory is an ability of the central nervous system to fix, keep and as necessary to reproduce information on knowledge...
Section: Articles about health
According to data of World Health Organization, the cataract is diagnosed almost for 7% of the population of Earth. The statistics of incidence is considered not full as at an initial stage the illness, as a rule, does not cause to the person of special inconveniences, and many having got sick...
Section: Articles about health
Long time antibiotics were considered as a panacea from all diseases and were appointed even at insignificant symptoms of an infection. Even now not everyone knows in what force of antibiotics how and when they should be accepted. Let's discredit 7 popular myths about such drugs....
Section: Articles about health
Scientists have no unambiguous opinion on a proximate cause of emergence of a carcinoma cutaneum today. Are precisely established only фа...
Section: Articles about health
People know that thermal sources have salutary force long ago. Treatment by natural waters is one of the most ancient methods of disposal of the most different diseases. Bathtubs, souls, wrappings and inhalations, in combination with reception of water vnut...
Section: Articles about health
Traveling all over the world, many try to try the most exotic dishes of national cuisines. There is even a so-called gastronomic tourism which, according to gourmets, not only allows to receive new feelings, but also is capable to show life of other people from absolutely unexpected side....
Section: Articles about health
The varicosity has familiarly many, statistically, this disease more than a half of all adult population. As...
Section: Articles about health
The trophic ulcer is not an independent disease. This heavy complication arising owing to a thermal injury (a burn or a frostbite), chronic pathologies of arteries or veins of the lower extremities, a diabetes mellitus, and also some defeats of a soyeda...
Section: Articles about health
Tea is loved and use almost everything. This drink has tonic properties, contains the tannins capable to suppress activity of causative organisms. Recently great popularity was gained by teas with vegetable additives. The medicative herbs, spices and fruit which are a part of such mixes enrich drink with vitamins and microelements, increasing its nutritional value and creating additional curative effect....
Section: Articles about health
Sometimes it seems that modern society was divided into two camps: representatives of the first are sure that has to for contraception отвеч...
Section: Articles about health
From the failure of work of immune system which is shown in the form of an allergy, statistically, more than 40% of the population of the globe suffer. In most cases pathological reactions cause the substances which are contained in food stuffs, hair of animals, medicines...
Section: Articles about health
The summer of this year in Russia was very ambiguous. Regions suffered from a merciless heat, from pouring rains, the hail from time to time dropped out, then there was again a heat which alternated with rainfall again. Many people suffer from such sharp changes of weather. Even flu epidemics and a SARS were recorded....
Section: Articles about health
The fatigue, sleep debt, disturbances of food, bad mood, vagaries of the weather – all these circumstances badly are reflected in our vn...
Section: Articles about health
The popular expression "run from a heart attack" became the motto of the people supporting active lifestyle. Moreover, run became a peculiar fashionable tendency: sales of racetracks and the accompanying goods for run are at permanently high level. Really...
Section: Articles about health
Popular joke that there are no healthy people, and is nedoobsledovanny, most of us considers an honest truth, continually it is necessary to hear that all of us are sick hardly from a school bench. It is hard to say whether so it actually because too often people are treated for nonexistent diseases, and sometimes call a disease what is something another. Sometimes in it the doctors of old school making diagnoses which are cancelled long ago – medicine still unless are guilty...
Section: Articles about health
Statistically, in Russia about 34% of citizens smoke. Most of consumers of tobacco has problems about health sooner or later...
Section: Articles about health
Proofs of efficiency of Mildronate at treatment of coronary heart disease with stenocardia can be found in many publications of the end of the twentieth century. Researches were conducted since 1984, including placebo - controlled effects. In total клиничес...
Section: Articles about health
Practice of hypnotic impact on consciousness of the person contains about two millennia. During this time scientists managed to learn a lot of things about a phenomenon of hypnosis and learned to facilitate a condition of the patients having heavy illnesses with its help....
Section: Articles about health
The drugs stopping or oppressing life activity of pathogenic microorganisms are widely applied in clinical practice with 4...
Section: Articles about health
Iodine - one of thirty most important microelements in our organism. The main role of iodine consists in synthesis of thyroid hormones of a thyroid gland - the substances which are responsible for the majority of exchange processes of an organism. It is known that thyroid hormones consist...
Section: Articles about health
The brain of the person is studied not one hundred years, but the quantity of the riddles connected with this body increases rather, than decreases. Perhaps, numerous delusions concerning a structure and functioning of a brain are explained by it, many of which arose long ago, but continue to exist and today. Such we are ready to acquaint readers with the most widespread myths....
Section: Articles about health
The state of health of the person in many respects depends on food. The organism will well function if during food it are...
Section: Articles about health
Venereal diseases in medicine are called the infections which are transmitted preferential sexually, now they and are called - infections, sexually transmitted, or STD. Among them is also life-threatening. In spite of the fact that majority...
Section: Articles about health
80% of women at least once to lives complained of discomfortable feelings to breasts, consolidations and nagrubaniye. These are mastopathy symptoms. The mastopathy is characterized by change of a ratio between ferruterous and connective tissue tissues of mammary glands. It can lead to formation of cysts (a cystous mastopathy), gland consolidation (a fibrous mastopathy), or a combination of these processes (a fibrous and cystous mastopathy)....
Section: Articles about health
Life expectancy in various regions of Earth is not identical. Exert impact on it social stability, economic бл...
Section: Articles about health
Several decades ago the basil (the district khan, реан, Reagan) was considered as a part of the Caucasian or east cuisine, but today it strongly took the place on tables of Russians. Greens of this plant possess a strong, pleasant smell and specific fresh taste, because of to...
Section: Articles about health
For the help to doctors in the choice of optimal solutions for treatment of various diseases the Cochrane scientific organization (Cochrane) conducts joint researches with representatives of scientific community around the world. The analysis of a series of the conducted researches of the drug Oscillococcinum® relating to group of cold remedies became one of the last methanolyses....
Section: Articles about health