





Sodium hydroxide
Sodium hydroxide (E524 nutritional supplement, caustic natron, sodium hydroxide, the caustic soda) – the firm alloyed mass of yellowish or white color. On the  chemical properties sodium hydroxide belongs to strong alkali.
chemical properties sodium hydroxide belongs to strong alkali.
General properties of sodium hydroxide
Caustic natron usually is produced in the form of transparent colourless solution or in the form of paste.
The caustic soda is perfectly dissolved in water, generating heat. At interaction with air this substance spreads therefore it goes on sale in a hermetically sealed container. In nature the hydroxide of sodium is a part of mineral of brucite. Temperature of boiling of a hydroxide of sodium makes 1390 °C, melting temperature – 322 °C.
Receiving sodium hydroxide
In 1787 the doctor Nikola LeBlanc developed convenient metol of receiving sodium hydroxide from chloride sodium. Later LeBlanc's method was forced out by an electrolytic way of receiving caustic natron. In 1882 the ferritic way of receiving sodium hydroxide based on use of the calcinated soda was developed.
Now sodium hydroxide is received most often by electrolysis of saline solutions. The ferritic way of receiving the caustic soda is now used rather seldom.
Sodium hydroxide use
Sodium hydroxide – incredibly popular and widely used chemical compound. About seventy million tons of caustic natron are annually produced.
The caustic soda is used in the pharmaceutical, chemical, food industry, and also in cosmetic and textile. Caustic natron is applied at production of synthetic phenol, glycerin, organic dyes, medicines. This connection can neutralize the components, harmful to a human body, which are contained in air. Therefore sodium hydroxide solutions quite often use for disinfection of rooms.
In the food  industry the hydroxide of sodium is used as the acidity regulator interfering balling up and caking. E524 nutritional supplement supports a necessary consistence of products by production of margarine, chocolate, ice cream, butter, caramel, jelly, jam.
industry the hydroxide of sodium is used as the acidity regulator interfering balling up and caking. E524 nutritional supplement supports a necessary consistence of products by production of margarine, chocolate, ice cream, butter, caramel, jelly, jam.
Bakery products before pastries process solution of the caustic soda for receiving a dark brown crisp. Besides, E524 nutritional supplement is applied to refinement of vegetable oil.
Harm of sodium hydroxide
Caustic natron – the toxic destroying a mucous membrane and integuments. Burns from sodium hydroxide very slowly heal, leaving hems. Hit of substance in eyes most often leads to sight loss. At hit of alkali on integuments it is necessary to wash out the struck areas a water stream. At hit in an organism caustic natron causes burns of a throat, oral cavity, stomach and gullet.
All works with a hydroxide of sodium should be carried out in goggles and in overalls.
Was considered earlier that yawning enriches an organism with oxygen. However this opinion was disproved. Scientists proved that yawning, the person cools a brain and improves its working capacity.
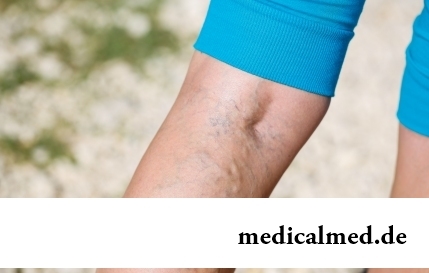
The varicosity has familiarly many, statistically, this disease more than a half of all adult population. As...
Section: Articles about health
Women quite often suffer from complexes concerning the sizes of the bust. Strangely enough, not too modest, and excessively curvy shapes become the reason of sincere discomfort sometimes. Except psychological problems, a big bust sometimes with...
Section: Articles about health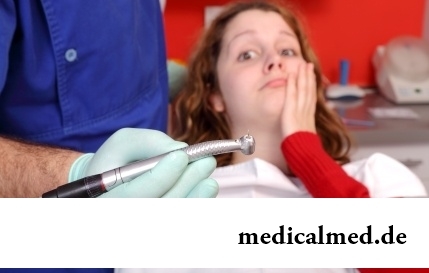
Statistically, can only one of ten of our compatriots brag of a decent condition of an oral cavity. Six teeth affected with caries are the share of the average Russian. For comparison, this indicator for Europeans is almost six times less....
Section: Articles about health
Heart disease and blood vessels lead to disturbance of blood supply of bodies and fabrics that involves failures in their works...
Section: Articles about health
Long time antibiotics were considered as a panacea from all diseases and were appointed even at insignificant symptoms of an infection. Even now not everyone knows in what force of antibiotics how and when they should be accepted. Let's discredit 7 popular myths about such drugs...
Section: Articles about health
The mankind knows that some toxins at intake in the minimum quantities have therapeutic effect from an extreme antiquity. Many substances recognized poisonous are applied in the medical purposes also today, being the main operating components of the medicines which are officially produced by pharmaceutical industry. Let's tell only about the most known of them....
Section: Articles about health
Let's begin with the fact that a separate illness which is called "adjournment of salts", just does not exist. In practice this household name of plank beds...
Section: Articles about health
The concept "gluten" (differently, a gluten) combines group of the proteins which are a part of rye, barley and wheat. For most of people the use of the food stuffs containing a gluten not only is safe, but also it is very useful. Nevertheless, there is a number the myth...
Section: Articles about health
The person, as well as all other beings living on our planet feels weather changing. It is the normal meteosensitivity which is not causing to healthy people of special troubles. Meteodependence, on the contrary, is the morbid condition which is characterized by an exacerbation of chronic illnesses at change of air temperature, differences of atmospheric pressure, wind strengthening, magnetic storms and other "surprises" on which the nature is so generous. The people suffering from meteodependence have to з...
Section: Articles about health
Dark circles (bruises) under eyes – a shortcoming with most of which often fight against the help of cosmetics (proofreaders, salons...
Section: Articles about health
Stability of a hormonal background is one of the most important conditions of preservation of health of the woman. At the same time endocrine system – the thin device extremely sensitive to any external influences. Changes of an image жиз can become the reason of hormonal failure...
Section: Articles about health
You are office worker, the driver, the fan of winter sports or do not think of life without bicycle? You lead a slow-moving life and you move on the city only on the car? You have no constant partner and you do not love the protected sex? Attention! You unambiguously are a potential target for prostatitis. It is not necessary to panic, it is necessary to work....
Section: Articles about health
Household skills which to us so diligently imparted in the childhood it appears, not always bring only benefit. According to result...
Section: Articles about health
From sexual contacts each person can test insufficiently strongly expressed sexual desire or lack of satisfaction from time to time. However when it happens regularly, it is an occasion to think about health. Most of people does not hurry an obrashcha...
Section: Articles about health
The advantage of swimming for the person is so high that this sport is not only the most popular, but also is widely applied in medicine and rehabilitation processes. If you look for for yourself the occupation allowing pleasantly and to spend time, then swimming with advantage – the fact that it is necessary for you. And give learns several facts about swimming....
Section: Slideshow
Each of us repeatedly noticed that the people having the same passport age are sometimes not similar on one-years at all. One...
Section: Articles about health
Partial and the more so full loss of hearing significantly reduces quality of life. Difficulties with communication lead to loneliness and isolation. The person who badly hears experiences difficulties with social and professional implementation, quite often has problems in...
Section: Articles about health
Statistically, in Russia about 34% of citizens smoke. Most of consumers of tobacco has problems with health sooner or later. Not only smokers, but also their relatives suffer. Besides, cigarettes are expensive, and need of their acquisition goes a heavy burden on the budget of thousands of Russian families. Many people dream to refuse harmful tendency, but everyone manages to make it not: nicotine addiction is affectionate and to get rid of it not easy....
Section: Articles about health
Mushrooms - the surprising inhabitants of our planet having a set of wonderful qualities. Thanks to one of them, a mold mushroom of Penici...
Section: Articles about health
Neurosis is called pathology of a nervous system at which deviations in functioning of the highest nervous processes are observed. Most often - owing to yet not strengthened mentality - children are subject to neurosises. Premises to emergence of such disturbances can become нез...
Section: Articles about health
Producers of milk mixes for children assure: mixes are ideally balanced and adapted for needs of babies. If mother should raise artificially the kid owing to serious problems with health, to do nothing – it is necessary to feed with substitutes of milk. However pediatricians note that not seldom women without good reasons refuse feeding of the child a breast and pass to milk mixes. Common causes of such decision – the aspiration to leave quicker...
Section: Articles about health
Sometimes it seems that modern society was divided into two camps: representatives of the first are sure that has to for contraception отвеч...
Section: Articles about health
The sudden heat on all body which is followed by perspiration and a cardiopalmus – the phenomenon familiar to many people. Most often such states called by "inflows" result from nervous or physical overworks and disappear right after rest. Odn...
Section: Articles about health
History of cultivation of a buckwheat contains more than five thousand years. Grain which is received from this plant is used for preparation of porridges, soups, baked puddings and puddings, do flour which is one of the main ingredients of the noodles popular in many countries of it. Buckwheat dishes are useful and tasty, they are perfectly combined with meat, milk, eggs, mushrooms, fruit and vegetables....
Section: Articles about health
Several decades ago the basil (the district khan, реан, Reagan) was considered as a part of the Caucasian or east cuisine, but today it is strong for...
Section: Articles about health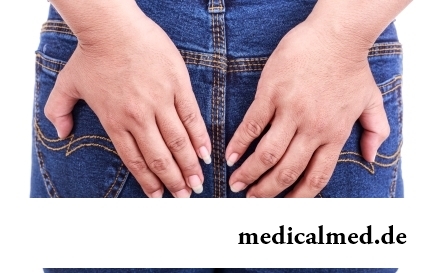
Hemorrhoids – extremely widespread disease. Periodically arising inflammations and bleeding of hemorrhoidal nodes cause serious discomfort to nearly fifteen percent of adults. Meanwhile, having a clear idea of the aggravation reasons...
Section: Articles about health
Traveling all over the world, many try to try the most exotic dishes of national cuisines. There is even a so-called gastronomic tourism which, according to gourmets, not only allows to receive new feelings, but also is capable to show life of other people from absolutely unexpected side....
Section: Articles about health
