





Orthophosphoric acid
Orthophosphoric acid is inorganic acid of average force.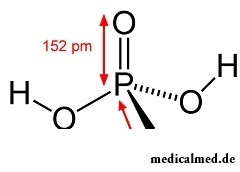
Its chemical formula – H3PO4, designation as nutritional supplement – E338.
Orthophosphoric acid represents colourless hydroscopic crystals, well soluble in water (as a rule, orthophosphoric acid call its 85% solution).
Receiving orthophosphoric acid
Receiving orthophosphoric acid happens:
- Phosphorus pentachloride hydrolysis;
- From phosphate;
- Interaction of oxide of phosphorus (V) with water.
Properties of orthophosphoric acid
The main property of orthophosphoric acid is its impact on acid-base balance of an organism that leads to increase in acidity.
The hyperoxemia increases risk of development of many diseases, including caries and early osteoporosis.
One more property of orthophosphoric acid (in high concentration) is its ability to cause burns. Vapors of this acid can cause nasal bleedings and atrophic processes in mucous a nose, and in certain cases can lead to destruction of teeth and change of a blood count.
Interaction of orthophosphoric acid
Interaction of orthophosphoric acid happens with:
- Metals;
- Main oxides;
- Bases;
- Ammonia;
- Salts of weak acids.
Use of orthophosphoric acid
Orthophosphoric acid is widely used in various purposes:
- For clarification from a rust of metal surfaces. Also it promotes further prevention of formation of corrosion;
- As gumboil at the soldering;
- In agriculture – for production of mineral fertilizers;
- As a part of freon (in industrial freezing installations);
- For researches in the field of molecular biology;
- In production household synthetic cleaning, washing and emollients;
- In the aviation industry (as a part of hydroliquids).
Use of orthophosphoric acid in medicine
Orthophosphoric acid is widely applied in stomatology when sealing teeth. It pickle an adamantine substance of tooth before the procedure.
The main complexity is the impossibility to check depth and extent of demineralization of dentine and enamel, and also its full removal before sealing. The remains of orthophosphoric acid can lead to decrease in durability of a bonding and formation of "an acid mine".
Also in insignificant quantities orthophosphoric acid is applied in compositions of bleaches to teeth.
Use of orthophosphoric acid (E338) in the food industry
E338 (orthophosphoric acid) concerns to group of antioxidants – the additives protecting food stuffs from oxidation and discoloration.
Orthophosphoric acid is applied to acidulation of food stuffs and drinks. It provides acute or acid taste. From natural podkislitel, for example, of citric acid, it is distinguished the low cost and ease of receiving that allows to use widely it at production:
- Baking powders;
- Processed cheeses;
- Sausages;
- Sahara;
- The flavored drinks – Coca-Colas, Pepsi Cola, sprite.
Though orthophosphoric acid as nutritional supplement is allowed for use in the majority of the countries, as a result of many researches its harmful effects on an organism were established. So, it breaks acid-base balance, increasing its acidity. It can provoke development of various diseases, for example, of caries and osteoporosis.
The frequent use of orthophosphoric acid as a part of various products can cause digestion work disturbances – vomiting, nausea. Also prolonged use of products with this nutritional supplement can lead to a fastidium and loss of weight.
When lovers kiss, each of them loses 6,4 calories a minute, but at the same time they exchange nearly 300 species of various bacteria.

Practically each person is familiar with the annoying, pulling, unscrewing pains caused by overcooling of muscles of a back. In некото...
Section: Articles about health
The popular expression "run from a heart attack" became the motto of the people supporting active lifestyle. Moreover, run became a peculiar fashionable tendency: sales of racetracks and the accompanying goods for run are at permanently high level. Really...
Section: Articles about health
You heard that laughter prolongs life? Researchers did not manage to establish longevity direct link with sincere fun yet, but several facts confirming beneficial influence of risibility on the state of health are clinically proved....
Section: Articles about health
Zone hypostases under eyes - very widespread problem giving to people is a lot of inconvenience. Hypodermic fabric in these parts having...
Section: Articles about health
About 10-15 years ago existence of the computer in the apartment of the Russian was considered as a rarity and office rooms were only at the first stage of equipment by these useful devices. Today practically in each house there is a computer (and often not one), and to constants...
Section: Articles about health
A little more than a century ago goat milk was a traditional food stuff of most of Russians. Unfortunately, today on tables of our compatriots it appears extremely seldom. The reason that the use of so useful product practically came to naught, not only in very modest volumes of its production and, respectively, rather high cost. Potential consumers are just insufficiently informed on unique properties of goat milk and that advantage which...
Section: Articles about health
No, probably, the person who would not have cold. Cold, cough, a headache – these symptoms are known to everyone. Peak to Prost...
Section: Articles about health
The chia plant, or the Spanish sage, is from South America. The indigenous people of the continent since ancient times used its seeds in food: small, but very nutritious kernels, in a form the reminding fasolina. Indians knew about useful properties of seeds of a chia, and applied...
Section: Articles about health
Condition of lips (their morbidity, outward) – one of indicators of health of the person. The peeling, dryness, pallor, and also cracks in corners of a mouth can be not only the cosmetic shortcoming which arose owing to physical damages and weather conditions but also the satellite of some diseases and disturbances in an organism needing treatment. Let's consider 10 possible reasons of emergence of angular cracks (perleches) in corners of a mouth and ways of their elimination....
Section: Articles about health
Kidneys perform the most important function of clarification of blood from those products of metabolic processes which cannot be used орг...
Section: Articles about health
Reactive pancreatitis - the disease which is characterized by inflammatory process in a pancreas which arises most often because of excess activity of digestive enzymes. It − the emergency state which treatment has to take place in хирургич...
Section: Articles about health
The brain of the person is studied not one hundred years, but the quantity of the riddles connected with this body increases rather, than decreases. Perhaps, numerous delusions concerning a structure and functioning of a brain are explained by it, many of which arose long ago, but continue to exist and today. Such we are ready to acquaint readers with the most widespread myths....
Section: Articles about health
Cold, puffiness of a nose, itch, the watering eyes - characteristic symptoms of the allergic rhinitis resulting from hit and...
Section: Articles about health
The stroke is one of the most widespread diseases of the person, annually in the world about 6 million cases of this pathology are registered. According to medical statistics, strokes occur almost three times more often than myocardial infarctions. Disease otno...
Section: Articles about health
Eyes – unique body on the structure thanks to which the person obtains about 80% of information on the world around: about a form, color, size, the movement, and also many other parameters of objects or phenomena. But whether much we know about the most valuable sense body which, according to the scientist Sechenov, provides us about one thousand various feelings a minute? Let's consider 10 most surprising facts about eyes and sight....
Section: Articles about health
80% of women at least once to lives complained of discomfortable feelings to breasts, consolidations and nagrubaniye. These are mastopathy symptoms. Mas...
Section: Articles about health
History of use of an anesthesia during operations contains more than 160 years. Annually in the world hundreds of thousands of surgical interventions during which to patients the substances immersing them in a dream and saving from pain are entered are carried out. Using an anesthesia to these...
Section: Articles about health
Physical activity is necessary for normal functioning of a human body. At a lack of the movement joints cease to function, muscles atrophy, cardiovascular activity is broken and the metabolism worsens. The modern city rhythm of life does not provide the person with an adequate exercise stress, additional - sport is necessary. Tedious tasks the huge number of the people having those or ин exists sport not all to liking, but also...
Section: Articles about health
Such trouble as the milkwoman's attack, at least once in life happened almost to each woman. Prevalence забол...
Section: Articles about health
For anybody not a secret that our country is one of the most "drinking" in the world. At clear understanding that the use of hard alcoholic drinks – occupation extremely harmful, most of Russians belong to alcoholism with unjustified loyalty. These...
Section: Articles about health
Any person who faced a disease knows that treatment costs expensive. It belongs also to consultations of qualified specialists, and to the diagnostic procedures which are not included in the list of obligatory medical services. The question of cost of medicines is not so unambiguous: almost each drug is produced several producers at once, and the price of medicine can differ many times. In such situation there is a sense to understand in what differ from each other original environments...
Section: Articles about health
There is an opinion that at low temperatures safety of products is ensured longer and better thanks to what the refrigerator considers...
Section: Articles about health
Ability of an organism to resist to adverse environmental factors (to impact of temperature drops, humidity and pressure, to the attacks of causative organisms, etc.) directly depends on what the person eats. Business here not only in, that C...
Section: Articles about health
From the failure of work of immune system which is shown in the form of an allergy, statistically, more than 40% of the population of the globe suffer. In most cases pathological reactions cause the substances which are contained in food stuffs, hair of animals, medicines, goods of household chemicals, cosmetics, pollen of plants, etc. On the one hand, the disease such is capable to spoil quite thoroughly to the person life....
Section: Articles about health
Antibiotics - - it is possible to call the chemical compounds suppressing growth of bacteria the break in the field of medicine which allowed to save persons...
Section: Articles about health
Diapers for adults – individual one-time means of hygiene which in some situations is irreplaceable and from such situations any person is not insured. Though nobody perceives need of their use with enthusiasm, however without it to a sra...
Section: Articles about health
Bulimia and anorexia, are heavy deviations of a feeding behavior, become a cause of death of patients much more often than all other nervous breakdowns combined. In 60% of cases two illnesses accompany each other: patients feel horror before danger of set of excess weight and try to refuse as often as possible food, but periodically suffer from attacks of sudden hunger and an uncontrollable overeating. Each patient with anorexia and bulimia needs the help qualified пс...
Section: Articles about health
