





Авонекс
Application instruction:
 Авонекс – interferon, drug for treatment of multiple sclerosis.
Авонекс – interferon, drug for treatment of multiple sclerosis.
Form of release and structure
- Lyophilisate for preparation of solution for intramuscular introduction: the mass of almost white or white color (in the glass bottles closed by brombutilovy traffic jams and the Bio-Set device, 1 bottle and solvent (the glass syringe) in the soldered plastic tray, in a pack cardboard 4 trays);
- Solution for intramuscular introduction: transparent, colourless (on 0,5 ml in the glass syringes closed by brombutilovy caps, Luer Lock devices and a polypropylene stopper plunger with a capacity of 1 ml, 1 syringe with a needle in the soldered plastic tray, in a pack cardboard 4 trays).
Active agent: interferon beta 1а, its contents in 1 bottle of lyophilisate and 1 syringe with solution makes 30 mkg (6 million international units).
Auxiliary components of lyophilisate: albumine human serumal, sodium hydrophosphate, sodium dihydrophosphate and sodium chloride.
Composition of solvent: water for injections.
Solution excipients: water for injections, arginine a hydrochloride, sodium acetate trihydrate, polysorbate 20, glacial acetic acid.
Indications to use
- Recurrent multiple sclerosis in case of at least two aggravations within the previous 3 years in the absence of signs of progressing of a disease between a recurrence (for delay of progressing of functional disturbances and decrease in frequency of a recurrence);
- Clinically isolated demyelination case in the presence of the active inflammatory process which demanded intravenous administration of glucocorticosteroids if others are excluded, than multiple sclerosis, states and is available high risk of its development.
Contraindications
Absolute:
- Epilepsy steady against therapy;
- The expressed depression;
- Emergence of suicide thoughts;
- Period of pregnancy and lactation;
- Age up to 18 years – for solution, up to 16 years – for lyophilisate;
- Hypersensitivity to natural or recombinant interferon a beta, to auxiliary components of drug or seralbumin of the person.
With care:
- Heavy renal/liver failure;
- The expressed oppression of a marrowy hemopoiesis;
- Convulsive attacks in the anamnesis;
- Depressive frustration;
- Heart diseases (arrhythmia, stenocardia, congestive heart failure, the postponed myocardial infarction).
Route of administration and dosage
Авонекс it is intended for intramuscular introduction. Treatment is carried out under control of the doctor which has experience of treatment of the multiple sclerosis (MS).
The recommended dose makes 30 mkg once a week. The injection whenever possible should make at the same time on the same day weeks. The place of an injection needs to be changed every week.
From lyophilisate prepare solution for intramuscular introduction just before an injection.
For reduction of frequency of development and weight of grippopodobny symptoms at an initial stage of therapy titration of a dose can be carried out: treatment is begun about ¼ doses and every week it is increased on ¼ until the full dose is reached (by fourth week).
Avoneks's introduction according to the alternative scheme is possible: treatment is begun about ½ doses once a week, then it is raised to a full dose.
To reduce expressiveness of grippopodobny reaction, before an injection and within 24 hours after each introduction it is recommended to accept a febrifugal analgetic.
Duration of treatment is defined individually. 2 years later after the beginning of therapy of the patient direct to clinical inspection and assessment of the neurologic status on the basis of which results the doctor will make the decision on need of continuation of a course. In case of development of the chronic progressing RS drug should be cancelled.
Rules of use of solution for injections:
- Before introduction it is necessary to extract drug from the refrigerator and to leave it at the room temperature (15-30 ºС) approximately for 30 minutes;
- It is impossible to use external sources, including hot water for solution warming;
- Not to use drug if it contains solid impurity, or is not transparent and colourless;
- Each syringe is expected one injection. The unused amount of solution should be utilized.
Rules of preparation of solution for injections from lyophilisate:
- To check integrity of a bottle and the Bio-Set device (if it is broken, drug is forbidden to be used);
- Having taken for foundation of Bio-Set, to turn a cap and to remove it, without touching a connecting opening;
- From the syringe with solvent to remove a cap (to pull together), without touching a tip and without pressing the piston;
- To put a bottle vertically on a smooth surface, to combine with a syringe tip. To screw clockwise a syringe cannula in Bio-Set. Holding the syringe for the basis and following the direction of the movement, sharply its tax down that the tip completely disappeared and the click sounded;
- Slowly pressing on the syringe piston, to enter solvent into a bottle;
- Without separating the syringe with Bio-Set, to carefully rotate a bottle until powder completely is dissolved (about 1 minute). Solution, ready to use, has to be colourless (or with a subtle yellowish shade) and transparent, not to have mechanical impurity. It is not necessary to stir up a bottle since foaming is possible;
- To press on the syringe piston down to an emphasis to remove air;
- To turn a bottle and the syringe vertically on 180 º and to pull slowly for the piston to gather solution in the syringe;
- Without removing a cap from a needle, to open its individual packaging;
- Holding the syringe for the basis and not concerning its cannula, to separate the syringe from the Bio-Set device turn counterclockwise;
- Turning a needle clockwise, to get it on the filled syringe;
- To put the syringe on a plain surface.
Rules of administration of the solution for injections prepared from lyophilisate:
- To process the place of an injection the cotton plug moistened in alcohol;
- To remove a protective cap from a needle (without rotating, and pulling together);
- To turn the syringe a needle up;
- To remove air: to knock on the syringe basis that vials of air rose up, then to press slightly the piston until on the end of a needle the small droplet of liquid appears;
- To stick a needle in a muscle and to slowly administer the drug;
- To remove the syringe with a needle;
- In case of blood in the place of an injection to stick it with a plaster.
Important information:
- For preparation of solution it is possible to use only the solvent which is included in the package with lyophilisate;
- At connection of the syringe to the device it is impossible to take further actions, the click will not sound yet;
- It is not necessary to enter solvent too quickly since possibly foaming which will complicate a set of drug in the syringe.
Each bottle of Avoneks is intended for one injection. The remained drug is not subject to further use.
Side effects
The most frequent side effect of drug is the grippopodobny syndrome which is shown the following symptoms: fever, feeling of fatigue, a fever, weakness, a headache, muscular pains, nausea (are usually shown in an initiation of treatment and pass in process of use continuation). There are spastic phenomena and passing muscular weakness, up to reversible paralysis of extremities less often (these manifestations can pass and appear again, are usually short).
During any period of treatment there can be neurologic symptoms similar to an aggravation of a multiple sclerosis: the muscular weakness and/or episodes of muscular spasms limiting autokinesias (these episodes usually appear after drug injections, in certain cases are followed by grippopodobny symptoms).
The following side effects are less often observed:
- From the central and peripheral nervous system: headache, concern, decrease in sensitivity, sleeplessness, dizziness, paresthesias; seldom – migraine, a depression, confusion of consciousness, epileptopodobny attacks, emotional lability, psychosis, suicide thoughts;
- From cardiovascular system: arrhythmia, tachycardia, vazodilatation, feeling of heat, heart failure, cardiomyopathy;
- From system of a hemopoiesis: seldom – decrease in a hematocrit, a neutropenia, a lymphocytopenia, a leukopenia, thrombocytopenia, a pancytopenia;
- From respiratory system: rhinorrhea, asthma, диспноэ;
- From a musculoskeletal system: pain in extremities, a mialgiya, muscular spasms, an arthralgia, muscle tension, neck and spin pain, tranzitorny increase or decrease in a muscle tone in an initiation of treatment;
- From endocrine system: hypo - or a hyperthyroidism;
- From a reproductive system: metrorrhagias, menorrhagias;
- From the alimentary system: disturbances of functions of a liver, diarrhea, nausea, vomiting, appetite loss;
- Dermatological and allergic reactions: the strengthened sweating, an itch, rash, an alopecia, a small tortoiseshell, an exacerbation of psoriasis, anaphylactic reactions, an acute anaphylaxis;
- Others: change of body weight, sweating strengthening at night, a stethalgia, a hyperpotassemia, a system lupus erythematosus, tranzitorny increase in level of urea in blood serum;
- Local reactions: burning sensation, pain, an inflammation, a hyperemia, abscess in the place of an injection.
Special instructions
At Avoneks's appointment it is necessary to warn patients about need of the immediate address to the doctor in case of symptoms of a depression or suicide thoughts.
Except usual laboratory analyses which are carried out at multiple sclerosis during treatment it is necessary to carry out biochemical analysis of blood (including to check activity of enzymes of a liver), to define a leukocytic formula, to control a pattern of peripheral blood with calculation of uniform elements (including thrombocytes). In case of symptoms of a miyelodepressiya more careful blood analysis can be required.
During treatment by Avoneks in blood serum can appear interferon - the neutralized antibodies reducing activity of interferon of beta la and, as a result, efficiency of drug. According to the available data, 12 months of therapy of an antibody later to interferon beta 1а appear in serum approximately at 8% of patients.
Interferon beta 1а can exert a negative impact on the speed of reactions and ability to concentration of attention.
In case of pregnancy at women with high frequency of RS recurrence the doctor has to compare a ratio of risk of a heavy recurrence owing to Avoneks's cancellation and the increased probability of spontaneous abortion at continuation of its reception.
Medicinal interaction
Special researches on an occasion of interaction of Avoneks with other medicinal substances / drugs were not conducted. According to clinical data, patients with RS during an aggravation have beta interferon 1а it is possible to apply along with glucocorticosteroids or adrenocorticotropic hormone.
It is established that interferona can reduce activity of enzymes of system of P450 cytochrome therefore with care along with Avoneks it is necessary to use drugs which clearance substantially depends on this system, for example, antidepressants and antiepileptic means.
Terms and storage conditions
To store in the unavailable to children, protected from light place at a temperature: lyophilisate – no more than 25 ºС, solution – 2-8 ºС (not to freeze). Solution is allowed to be stored at a temperature of 15-30 ºС, but no more than 1 week.
Period of validity – 2 years.
The well-known drug "Viagra" was initially developed for treatment of an arterial hypertension.

Vitamin complexes belong to the most popular drugs, probably, in our country there is no person who was not hearing about a floor...
Section: Articles about health
Neurosis is called pathology of a nervous system at which deviations in functioning of the highest nervous processes are observed. Most often - owing to yet not strengthened mentality - children are subject to neurosises. Premises to emergence of such disturbances can become нез...
Section: Articles about health
The unpleasant feelings connected with spring breakdown are familiar almost to each of us. Often happens that in March-April on the person weakness leans: he suffers from drowsiness, complains of bad mood, loss of interest in life and failures in affairs....
Section: Articles about health
The state of health of the person depends on many factors. One of the most important is the constant but which is not exhausting, motive...
Section: Articles about health
Scientists always aimed to offer fundamental explanations for medical problems. Their theories formed the basis of modern methods of treatment of the hardest pathologies and helped to save a set of lives. However stories are known also such theoretical constructions, following to...
Section: Articles about health
Coffee - the tonic loved by many for the invigorating aroma and deep taste. Having the stimulating effect, coffee increases working capacity, promotes concentration of attention, fights against drowsiness and improves mood. Statistically, about 30% of inhabitants of the planet regularly use coffee, from them more than 8% are "coffee-achievers" - the persons using more than 3 cups of drink a day....
Section: Articles about health
Olive oil – the product capable to make a powerful contribution to health of the person if it includes it in the diet. Rich vitamin...
Section: Articles about health
Iodine - one of thirty most important microelements in our organism. The main role of iodine consists in synthesis of thyroid hormones of a thyroid gland - the substances which are responsible for the majority of exchange processes of an organism. It is known that thyroid hormones consist...
Section: Articles about health
Modern footwear is extremely various. It stopped being only protection for legs long ago. Today shoes, boots, barefoot persons choose not so much proceeding from their convenience and functionality how many being guided by outward, brand and an opportunity to add with them a stylish dress. At the same time, buying footwear, think of its safety a little. Meanwhile, many popular models can do essential harm to health....
Section: Articles about health
Wood louse – the ordinary-looking unpretentious plant extended in all territory of our country. It quickly expands, and sometimes for...
Section: Articles about health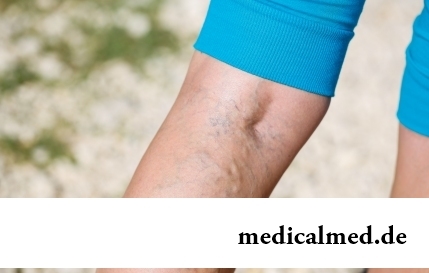
The varicosity has familiarly many, statistically, this disease more than a half of all adult population. As a rule, the varicosis affects preferential superficial vessels, and is shown by characteristic cosmetic defects. Guo...
Section: Articles about health
Each woman has preferences in the field of use of those goods which help us to look good, feel young and effective. Besides: selection process of favourite perfume, shampoo or decorative cosmetics already lightens the mood and serves as a peculiar stress medicine. Happens very offensively when the acquired perfumery and cosmetic products not only do not meet our expectations, but also becomes the reason of problems with health. Sources неприятн...
Section: Articles about health
Each person has easy indispositions which he transfers "standing", trying not to ask for medical care. Argu...
Section: Articles about health
Childbirth is the most important event in life of each woman. We are women we give birth to the new little man on this light. Now the tendency to that was outlined, as men want to participate in labor too. But there is a question and whether it is worth allowing the husband...
Section: Articles about health
Bulimia and anorexia, are heavy deviations of a feeding behavior, become a cause of death of patients much more often than all other nervous breakdowns combined. In 60% of cases two illnesses accompany each other: patients feel horror before danger of set of excess weight and try to refuse as often as possible food, but periodically suffer from attacks of sudden hunger and an uncontrollable overeating. Each patient with anorexia and bulimia needs the help qualified пс...
Section: Articles about health
The cosmetics intended for improvement of a condition of skin, nails and hair are used by each woman. Expenses on регуля...
Section: Articles about health
The summer of this year in Russia was very ambiguous. Regions suffered from a merciless heat, from pouring rains, the hail from time to time dropped out, then there was again a heat which alternated with rainfall again. Many people suffer from such sharp changes of weather...
Section: Articles about health
The pine is one of the most widespread plants of our woods. Its needles and pitch not without reason called by "gallipot" were since ancient times used for strengthening of protective forces of an organism, treatment of avitaminosis, anemia and many other diseases. In recent years wide popularity was gained by the national medicines prepared from pinecones. "Fruits" of a coniferous tree contain a huge amount of vitamins, biologically active agents, antioxidants, phytoncides and other useful to...
Section: Articles about health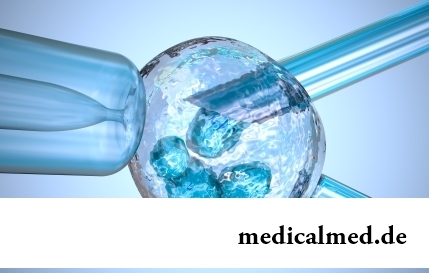
EKO, or extracorporal fertilization - a method of treatment of infertility which became the reason of a set broken mines in due time...
Section: Articles about health
Each person knows that fervescence is an illness sign. However too low temperature (hypothermia), especially also can demonstrate existence of diseases when it is observed long enough. Such state is dangerous those...
Section: Articles about health
Cold – a state known to everyone which is followed by cold, cough, high temperature, a pharyngalgia. Often the first that we begin to do in hope again to become healthy – to accept medicines which are not always harmless whereas it is easy to facilitate displays of a disease by means of natural means. They not only softly eliminate disease symptoms, but also enrich the weakened organism with useful substances. We present you 8 drinks which are successfully used for...
Section: Articles about health
Tuberculosis – a serious infectious disease which development is caused by mycobacteria (Koch's bacilli). The illness is known from a deep d...
Section: Articles about health
Is told about advantage of domestic animals for development of the child much. But many parents nevertheless do not hurry to bring pets as are afraid that they can do harm to health of children. What troubles can really trap kids and how to make with...
Section: Articles about health
The pancreas performs two functions in a human body: release of enzymes without which digestion of carbohydrates, fats and proteins, and a producing hormones is impossible. The most important of them - insulin, is the main participant of carbohydrate metabolism normalizing processes of education and utilization of glucose, the main energy source for an organism....
Section: Articles about health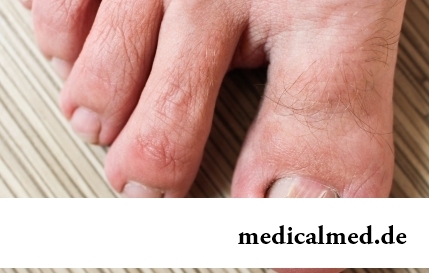
The word "onikhokriptoz" is unfamiliar to most of people, meanwhile quite so physicians call very widespread problem: growing...
Section: Articles about health
Stroke (acute disorder of cerebral circulation) – one of the most widespread neurologic diseases. Annually in the world more than 6 million people die of this illness. From the survived patients about 80% become disabled people, and nearly a thirds from them впо...
Section: Articles about health
The healthy nutrition is the invariable principle of health and good health for long years of the woman. Nevertheless, in a diet at each stage of life there are the features allowing to support an organism by those substances which are most necessary for it at present. Eating according to them, the woman will be able to feel vigorous and strong, and also to adapt to changes in an organism so that they allowed it to lead active lifestyle at any age....
Section: Articles about health
