





Kleksan
Application instruction:
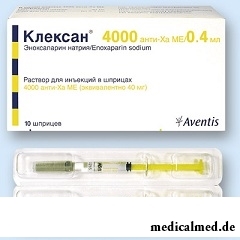 Kleksan – heparin low-molecular, anticoagulant of direct action.
Kleksan – heparin low-molecular, anticoagulant of direct action.
Form of release and structure
Kleksan is issued in the form of solution for injections: transparent liquid from pale yellow color to colourless (on 1 ml, 0,8 ml, 0,6 ml, 0,4 ml or 0,2 ml in glass syringes (type I) or in glass syringes (type I) with protective system of a needle, in blisters on 2 syringes, in a cardboard pack of 1 or 5 blisters).
Active ingredient – эноксапарин sodium, makes its contents in Anti-ha IU (International Units):
- 1 syringe on 0,2 ml – 2000;
- 1 syringe on 0,4 ml – 4000;
- 1 syringe on 0,6 ml – 6000;
- 1 syringe on 0,8 ml – 8000;
- 1 syringe on 1 ml – 10000.
Indications to use
- Therapy of a deep vein thrombosis at patients with a thromboembolism of a pulmonary artery or without it;
- Treatment of a myocardial infarction without tooth of Q and unstable stenocardia in a combination with acetylsalicylic acid;
- Therapy of an acute myocardial infarction with raising of a segment of ST at patients with exclusively drug treatment or subject to the subsequent transdermal coronary intervention;
- Prevention of thromboembolisms and venous thromboses at patients with chronic heart failure in a decompensation stage III or IV classes (functional classification of NYHA), an acute heart failure, heavy acute infections, acute rheumatic diseases against the background of one of the risk factors of a venous thrombogenesis, acute respiratory insufficiency forced to be on a bed rest;
- Prevention of venous embolisms or thromboses at surgeries, especially all-surgical and orthopedic interventions;
- Prevention of formation of blood clots during a hemodialysis lasting not more than 4 hours in system of extracorporal blood circulation.
Contraindications
- The diseases and clinical states interfaced to high probability of development of bleeding including a hemorrhagic stroke, the aneurism of vessels of a brain stratifying an aortic aneurysm (except surgery), the menacing abortion, heavy heparin - or the enoksaparin-induced thrombocytopenia, uncontrollable bleeding;
- Breastfeeding period;
- Age up to 18 years;
- Hypersensitivity to heparin, its derivatives and other low-molecular heparins.
Pregnant women with the artificial valve of heart are not recommended to use drug.
With care appoint Kleksan to patients with the following pathologies: a heavy vasculitis, disturbance of a hemostasis (including hemophilia, hypocoagulation, thrombocytopenia, an angiohemophilia), erosive cankers of the digestive tract (DT), including at an ulcer of a duodenum and stomach, the ischemic stroke (postponed recently), uncontrollable heavy arterial hypertension, a severe form of a diabetes mellitus, a hemorrhagic or diabetic retinopathy, a bacterial endocarditis (subacute or acute), a liver and/or renal failure, a pericardis or a pericardiac exudate, a severe injury (especially with damage of the central nervous system), extensive open wounds.
Besides the special attention is required by use at such situations as: the estimated or recently postponed ophthalmologic or neurologic surgical intervention, carrying out epidural or spinal anesthesia, recently postponed spinal puncture, intrauterine contraception, recent childbirth, the pregnancy period, a concomitant use of the means influencing system of a hemostasis.
There are no clinical data on Kleksan's use after recent performing radiation therapy and at patients with active tuberculosis.
Route of administration and dosage
Use of solution is made by deep hypodermic (п / to), intravenous (in/in) bolyusny injections or maintaining drug to the arterial site of the shunt in system of extracorporal blood circulation when carrying out a hemodialysis.
Contraindicated intramuscular administration of drug.
One-time syringes are ready to direct use.
The dose, way of introduction and the period of use are appointed by the attending physician on the basis of clinical testimonies and a condition of the patient.
The recommended dosing for п / to introduction:
- Prevention of venous embolisms or thromboses at surgeries: at all-surgeries – on 20 mg of 1 times a day, the first dose is entered in 2 hours prior to operation; at orthopedic and all-surgical interventions at patients with big risk of development of an embolism and thromboses – on 40 mg of 1 times a day, it is necessary to enter the first dose in 12 hours prior to operation, or on 30 mg 2 times a day, the first dose is entered 12-24 hours later after operation. The period of treatment of 7-10 days, in orthopedics – up to 5 weeks;
- Prevention of venous embolisms and thromboses at patients on a bed rest, at acute therapeutic diseases: on 40 mg of 1 times a day, a course of therapy of 6-14 days;
- Therapy of a deep vein thrombosis: on 1,5 mg on 1 kg of weight of the patient of 1 times a day or on 1 mg on 1 kg 2 times a day. It is desirable to carry out treatment in combination with indirect anticoagulants and to continue before achievement of indications 2-3 INR (the international normalized relation) in a blood koagulogramma, on average within 10 days;
- Treatment of a myocardial infarction without tooth of Q and unstable stenocardia: at the rate of 1 mg on 1 kg of body weight 2 times a day in a combination with acetylsalicylic acid in a dose of 100-325 mg of 1 times a day. Course of treatment of 2-8 days.
For prevention of a thrombogenesis in system of extracorporal blood circulation at a hemodialysis solution is entered into the arterial site of the shunt prior to the procedure in a dose from calculation for 1 mg on 1 kg of weight. For patients with high probability of development of bleeding dosing makes on 0,5 mg on 1 kg of weight in case of double vascular access or on 0,75 mg on 1 kg – at unary. One dose is expected a 4-hour session, at longer hemodialysis additional administration of solution from calculation for 0,5-1 mg on 1 kg of mass of the patient is allowed.
Therapy of a myocardial infarction with raising of a segment of ST should be begun with in/in bolyusny introductions of 30 mg of solution, then within the next 15 minutes п / to enter Kleksan in a dose 1 mg on 1 kg of body weight, the maximum dose of each of two first p / to injections can make 100 mg. The interval between all the subsequent п / to doses has to make 12 hours.
Treatment of patients at the age of 75 years is also more senior does not assume single in/in bolyusny introduction, to the patient appoint on 0,75 mg to 1 kg of weight with п / to introduction each 12 hours. Introduction of the first two doses of 75 mg of an enoksaparin of sodium irrespective of the patient's weight is allowed.
Therapy needs to be carried out with a concomitant use of acetylsalicylic acid in a dose 75-323 mg a day within a month. At a combination to trombolitika solution is recommended to be entered in 15 minutes prior to or in 30 minutes after thrombolytic therapy.
The period of use of drug at a myocardial infarction with raising of a segment of ST lasts 8 days.
In/in bolyusno drug administer through a venous catheter, Kleksan is compatible from 5% solution of a dextrose and 0,9% chloride sodium solution.
Mixing or introduction of an enoksaparin of sodium with other medicines is contraindicated.
At transdermal coronary intervention at patients with a myocardial infarction with raising of a segment of ST it is provided in bolyusny administration of drug in a dose of 0,3 mg on 1 kg of weight of the patient if from the moment of the last p / to an injection before inflating of a balloon catheter there passed more than 8 hours.
To patients of advanced age without renal failures, dose adjustment is not required, except for therapy of a myocardial infarction with raising of a segment of ST.
The recommended dosing for patients with a renal failure: at (п / to) use of drug with the medical purpose – on 1 mg on 1 kg of weight of 1 times a day; at treatment of an acute myocardial infarction with raising of a segment of ST at patients 75 years – single 30 mg, bolyusny in/in introduction, and п / to introduction of a dose from calculation for 1 mg on 1 kg of weight with the subsequent p / to introduction of the calculated dose of 1 times a day are younger; at treatment of an acute myocardial infarction with raising of a segment of ST at patients 75 years are more senior – without in/in bolyusny introductions to the patient appoint a dose from calculation for 1 mg to 1 kg of weight of 1 times a day. For each of the patients listed to category appointment of the first п / to an injection of 100 mg is allowed.
Preventive use of solution for patients with a renal failure is appointed п / to in a dose of 20 mg of 1 times a day.
Side effects
- From coagulant system of blood: very often – a hematoma, ecchymomas, nasal bleedings, a hamaturia, gastrointestinal bleedings, wound hematomas, a thrombocytosis at surgical patients and patients with a deep vein thrombosis with a thromboembolism or without it; often – nasal and gastrointestinal bleedings, ecchymomas, a hematoma, a hamaturia, wound hematomas at patients with unstable stenocardia, a myocardial infarction without Q tooth, a myocardial infarction with raising of a segment of ST and patients with heavy therapeutic pathologies on a bed rest, a thrombocytosis (patients with an acute myocardial infarction with raising of a segment have ST), thrombocytopenia at patients at prevention of venous thromboses at surgeries, a myocardial infarction with raising of a segment of ST and a deep vein thrombosis with a thromboembolism or without it; infrequently – intracraneal hemorrhages and retroperitoneal bleedings at patients with a deep vein thrombosis with a thromboembolism of a pulmonary artery or without it and at a myocardial infarction with raising of a segment of ST, thrombocytopenia – at the patients who are on a bed rest and at treatment of a myocardial infarction without tooth of Q and unstable stenocardia; seldom – retroperitoneal bleedings at patients at unstable stenocardia, surgeries, a myocardial infarction without Q tooth; very seldom – immuno-allergic thrombocytopenia at an acute myocardial infarction with raising of a segment of ST; frequency is unknown – development of a spinal or neuroaxial hematoma (against the background of spinal/epidural anesthesia or a spinal puncture);
- From system of a hemopoiesis: frequency is unknown – hemorrhagic anemia, immuno-allergic thrombocytopenia with thrombosis, a heart attack of bodies, ischemia of extremities, an eosinophilia;
- From immune system: often – allergic reactions; seldom – anaphylactic and anaphylactoid reactions; frequency is unknown – shock;
- From a nervous system: frequency is unknown – a headache;
- From a liver and biliary tract: very often – increase in activity of liver enzymes; frequency is unknown – hepatocellular and/or cholestatic damage of a liver;
- From a musculoskeletal system: frequency is unknown – osteoporosis (at therapy more than 3 months);
- From skin and hypodermic fabrics: often – an erythema, an itch, urticaria; infrequently – violent dermatitis; frequency is unknown – a purpura or erythematic papules, a skin vasculitis (in the place of an injection), an alopecia;
- Datas of laboratory: seldom – a hyperpotassemia;
- Others: often – pain, a hematoma, an inflammation, hypostasis in the place of an injection, hypersensitivity reaction, bleeding, formation of consolidations; infrequently – irritation in the place of an injection, a skin necrosis in the place of an injection.
Special instructions
Kleksan's use is accompanied by big risk of development of bleeding therefore it is necessary to diagnose it in time, to define the place of localization and to take emergency measures on stopping.
Therapeutic doses at patients of advanced age, especially at those who are more senior than 80 years pose a threat of development of bleedings therefore treatment of this category of patients should be carried out under careful observation.
In case of need simultaneous use of an enoksaparin of sodium with the drugs influencing a hemostasis, treatment needs to be accompanied with regular monitoring of laboratory indications and careful clinical observation. In the absence of special indications of this combination it is necessary to avoid.
At patients with the expressed renal failures it is always necessary to carry out dose adjustment, with easy or moderate deviations of creatinine clearance – careful control of a state is necessary.
Patients with low body weight (women less than 45 kg, men – 57 kg) have an increased risk of development of bleedings.
Use of drug for patients with obesity is connected with risk of development of thromboses and embolisms.
Enoksaparin of sodium can cause development of thrombocytopenia, usually it occurs at patients during the period about 5-21 days of use therefore it is recommended to carry out regular control of level of thrombocytes to blood in comparison with its indicators prior to treatment. In case of considerable (for 30-50%) decrease in level of thrombocytes – drug should be cancelled.
There is a big risk of development of persistent or irreversible paralysis during spinal or epidural anesthesia at Kleksan's use for patients in a dose higher than 40 mg, when using after operation of constant catheters, at simultaneous use of the means exerting impact on a hemostasis. The probability of complications is higher at the patients who transferred before operation or having deformation of a backbone, and also in case of repeated or traumatic than the carried-out spinal puncture. For decrease in risk of bleeding installation and removal of a catheter need to be carried out in 10-12 hours after the last use of drug in the dose recommended for prevention of a deep vein thrombosis. Administration of drug after removal of a catheter should be made in 2 hours. At impossibility of a dose decline of Kleksan the procedure of spinal or epidural anesthesia should be postponed.
At feeling of a dorsodynia, numbness or weakness in the lower extremities, disturbance of touch functions, function of a bladder and/or intestines the patient has to inform the doctor on emergence of these symptoms immediately. They are symptoms of a hematoma of a spinal cord and demand urgent treatment.
At observance of the doses provided for prevention of tromboembolic episodes, effect of drug has no significant effect on aggregation of thrombocytes, indicators of a blood coagulation and a bleeding time.
At development of an acute infection and serious rheumatic conditions use of an enoksaparin of sodium is justified if the specified pathologies proceed against the background of one of the following risk factors of a venous thrombogenesis: chronic respiratory insufficiency, malignant new growths, age is higher than 75 years, an embolism and fibrinferment in the anamnesis, hormonal therapy, obesity, heart failure.
Kleksan does not influence ability of the patient to control of vehicles and mechanisms.
Medicinal interaction
The probability of development of bleeding increases at simultaneous use of Kleksan with ketorolaky and other non-steroidal anti-inflammatory drugs, salicylates of systemic action, klopidogrely, tiklopidiny, acetylsalicylic acid, a dextran (with a molecular weight 40 kd), system glucocorticosteroids, anticoagulants, trombolitika, antagonists of a glycoprotein of IIb/IIIa and other antiagregant.
It is impossible to alternate use of solution of an enoksaparin of sodium to other low-molecular heparins.
Terms and storage conditions
To store in the place protected from light at a temperature up to 25 °C. To protect from children.
Period of validity – 3 years.
Name of drug
Price
Drugstore
Kleksan solution for инъ шпр 60 mg 0,6ml No. 2, Sanofi/Aventis
851 rub.
 Network of the Moscow drugstores of IFC
Network of the Moscow drugstores of IFCKleksan solution for инъ шпр 20 mg 0,2ml No. 10, Sanofi/Aventis
1690 rub.
 Network of the Moscow drugstores of IFC
Network of the Moscow drugstores of IFCKleksan solution for инъ шпр 40 mg 0.4ml No. 10, Sanofi/Aventis
2860 rub.
 Network of the Moscow drugstores of IFC
Network of the Moscow drugstores of IFCThe first vibrator was invented in the 19th century. It worked at the steam engine and intended for treatment of female hysteria.

Shops of household appliances offer us the huge choice of various devices for the house. Whether there are among this abundance devices which...
Section: Articles about health
Stability of a hormonal background is one of the most important conditions of preservation of health of the woman. At the same time endocrine system – the thin device extremely sensitive to any external influences. Changes of an image жиз can become the reason of hormonal failure...
Section: Articles about health
On health of the person physicians know about salutary action of animals long ago. About 7 thousand years ago great Hippocrates recommended to the patients riding walks for strengthening of a nervous system and increase in vitality....
Section: Articles about health
What is in our understanding weeds? It plants which are considered to be suitable only for compost pits and feeding жи...
Section: Articles about health
Aging — natural and inevitable process. Over time our skin loses elasticity, on it saggings are formed, the face form loses former clearness. The procedure of nitevy lifting (nitevy tightening) can successfully solve this problem. In order that it is better познако...
Section: Articles about health
Statistically, at the address to doctors seven of each ten patients complain of a headache. Actually it is much more people who are periodically feeling unpleasant feelings such. Many people, apart from a headache the reason for serious fears, prefer to muffle independently the next attack medicines. Such behavior is extremely careless, especially if this symptom appears regularly and is followed by other signs of an indisposition. Constants head Bol...
Section: Articles about health
History of cultivation of a buckwheat contains more than five thousand years. Grain which is received from this plant is used for пригото...
Section: Articles about health
The saying "the rich do not know how the other half lives" is known to all. In a broad sense it is that we can not always understand the person whose features of a state are unknown to us. If with physiological characters of diseases the situation is more or less clearly (having noticed and...
Section: Articles about health
Small appetite at the child – the complaint which pediatricians should hear practically from each mother. Most often it is carried to the category of children's whims, however the refusal of food in certain cases can be to alarming symptoms therefore it cannot be ignored....
Section: Articles about health
From the failure of work of immune system which is shown in the form of an allergy, statistically, more than 40% of the population of the globe suffer. In большинс...
Section: Articles about health
Practice of hypnotic impact on consciousness of the person contains about two millennia. During this time scientists managed to learn a lot of things about a phenomenon of hypnosis and learned to facilitate a condition of the patients having heavy illnesses with its help....
Section: Articles about health
The technique of acupuncture (acupuncture) is used in the medical purposes more than three and a half millennia. It is eurysynusic and recognized as official medicine in the majority of the developed countries of the world. Influence by fine needles on so-called points of acupuncture contributes to normalization of a metabolism and hormonal background, activates protective forces of an organism, has anesthetic and antiinflammatory effect, stabilizes a condition of mentality....
Section: Articles about health
Olive oil – the product capable to make a powerful contribution to health of the person if it includes it in the diet. Rich vitamin...
Section: Articles about health
Smoking not only exerts a negative impact on the state of health of the consumer of tobacco products, but is an air polluter the substances potentially dangerous to people around. In recent years significantly the number of people, стремящ increased...
Section: Articles about health
The summer of this year in Russia was very ambiguous. Regions suffered from a merciless heat, from pouring rains, the hail from time to time dropped out, then there was again a heat which alternated with rainfall again. Many people suffer from such sharp changes of weather. Even flu epidemics and a SARS were recorded....
Section: Articles about health
We live during an advertizing era. Daily each person receives a solid portion of persuasive councils about what to eat to be здо...
Section: Articles about health
Weakness of an ankle joint – very widespread problem. Its existence is demonstrated by tendency to a podvorachivaniye of legs when walking on heels, frequent painful sprains, pain on average and anonymous toes even after small nagruzo...
Section: Articles about health
Beauty shop – the place which is associated only with positive emotions: joy, pleasure, relaxation. However visit of salon where work with biological material of clients, not always harmlessly is conducted. Today more than 100 pathogenic microorganisms who can catch in beauty shop including deadly to health are known....
Section: Articles about health
Extracorporal fertilization – one of the most modern methods of controlling with infertility. So far it already helped znach...
Section: Articles about health
The popular expression "run from a heart attack" became the motto of the people supporting active lifestyle. Moreover, run became a peculiar fashionable tendency: sales of racetracks and the accompanying goods for run are at permanently high level. Really...
Section: Articles about health
Tea is loved and use almost everything. This drink has tonic properties, contains the tannins capable to suppress activity of causative organisms. Recently great popularity was gained by teas with vegetable additives. The medicative herbs, spices and fruit which are a part of such mixes enrich drink with vitamins and microelements, increasing its nutritional value and creating additional curative effect....
Section: Articles about health
Statistically, pathologies of a thyroid gland in the world more than 500 million people have. Failures in work of this body conduct to is heavy...
Section: Articles about health
Each person knows that fervescence is an illness sign. However too low temperature (hypothermia), especially also can demonstrate existence of diseases when it is observed long enough. Such state is dangerous those...
Section: Articles about health
According to data of World Health Organization, the cataract is diagnosed almost for 7% of the population of Earth. The statistics of incidence is considered not full as at an initial stage the illness, as a rule, does not cause to the person of special inconveniences, and many diseased sees doctors not at once. The cataract is not only one of the most widespread ophthalmologic illnesses, but also the reason of a half of cases of loss of sight....
Section: Articles about health
Hemorrhoids – extremely widespread disease. Periodically arising inflammations and bleeding of hemorrhoidal nodes пр...
Section: Articles about health
About 20% of the population of our planet have a hypertension (permanent increase in arterial pressure). This disease has an adverse effect on the standard of living, reduces working capacity, and in the absence of systematic treatment threatens with such complications as a heart attack...
Section: Articles about health
The body of the person almost for 60% consists of water. It is so important for normal functioning of an organism that loss of only one and a half percent of liquid already leads to the most unpleasant effects. The problems connected with deficit of water can overtake also the healthiest person if he, for example, spends several hours under the scorching sun, without having taken with themselves drink, but is very simple to correct health in this case. It is much more difficult to minimize effects of other reasons about...
Section: Articles about health

