Zantak
Application instruction:
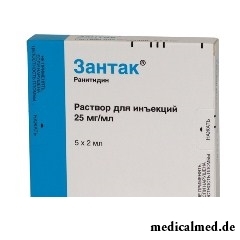
Zantak – antiulcerous means, a blocker histamine H2 receptors.
Form of release and structure
Dosage forms:
- Tablets, coated: a biconvex form, white color, with an engraving on one of the parties: on round – "GX EC2", on oval – "GX EC3" (on 10 pieces in blisters, in a cardboard pack 1 blister);
- Tablets are sparkling: from almost white till light yellow color, flat, round, with slanted edges (on 6 or 10 pieces in aluminum blisters, in a cardboard pack 1 or 2 blisters; on 15 pieces in polypropylene tubas, in a cardboard pack 1 tuba);
- Solution for injections: transparent liquid of light yellow color or colourless (on 2 ml in ampoules, in a cardboard pack of 5 ampoules).
Active ingredient of Zantak – ranitidine (in the form of a hydrochloride):
- 1 tablet, coated – 150 mg, 300 mg;
- 1 tablet sparkling – 150 mg, 300 mg;
- 1 ml of solution – 25 mg.
Auxiliary components:
- Tablets, coated: magnesium stearate, cellulose microcrystallic, methylhydroxypropyl cellulose, triacetin, titanium dioxide; besides, in oval tablets – sodium of a kroskarmelloz;
- Tablets are sparkling: Natrii hydrocarbonas, sodium monocitrate anhydrous, aspartame, Natrium benzoicum, K30 povidone, grapefruit fragrance, orange fragrance (content of sodium in 1 tablet – 328 mg or 479 mg respectively);
- Solution for injections: potassium dihydroortho-phosphate, sodium chloride, nitrogen, sodium hydroortho-phosphate disubstituted anhydrous, water for injections.
Indications to use
- The duodenum ulcer connected with Helicobacter pylori infection;
- The ulcer of a duodenum and high-quality expressions of a stomach including which appeared against the background of reception of non-steroidal anti-inflammatory drugs (NPVS);
- Gastroesophageal reflux disease, including stopping of a pain syndrome;
- Postoperative ulcers;
- Reflux esophagitis;
- The chronic incidental dyspepsia proceeding with the retrosternal or epigastric pains (which are not relating to above-mentioned states) which interrupt a sleep or are connected with meal;
- Zollingera-Ellison's syndrome;
- Prevention of stressful stomach ulcers at patients with a serious illness;
- Prevention of ulcers of a duodenum at use of NPVS (including acetylsalicylic acid), especially at a peptic ulcer in the anamnesis;
- Prevention of a recurrence of bleeding from round ulcers;
- Prevention of aspiration of acid contents of a stomach during the general anesthesia (Mendelssohn's syndrome).
Contraindications
- Acute porphyria (including the anamnesis);
- Period of pregnancy and breastfeeding;
- Age up to 12 years;
- Hypersensitivity to drug components.
It is necessary to appoint with care Zantak sick with a liver and renal failure, cirrhosis with portosistemny encephalopathy in the anamnesis.
Route of administration and dosage
Tablets, coated and tablets sparkling
Pill is taken orally.
The recommended dosing for adult patients:
- Exacerbation of benign stomach ulcer and duodenum: on 150 mg 2 times a day (in the morning and in the evening) or 300 mg of 1 times a day (for the night) within 4 weeks. In the absence of full scarring of an ulcer treatment should be prolonged for 4 weeks. For treatment of an ulcer of a duodenum the dose of 300 mg 2 times a day is more effective (increase in a dose does not influence the frequency of emergence of undesirable effects);
- Long prevention of a recurrence of stomach ulcer and duodenum: on 150 mg of 1 times a day (for the night), for the smoking patients the dose is raised to 300 mg of 1 times a day;
- The ulcers connected with reception of NPVS: on 150 mg 2 times a day or 300 mg of 1 times a day (for the night). A course of treatment – 8-12 weeks;
- Prevention of ulcers at use of NPVS: on 150 mg 2 times a day during the entire period of reception of NPVS;
- The duodenum ulcer associated with Helicobacter pylori: on 150 mg 2 times a day or 300 mg of 1 times a day in a combination from 750 mg of amoxicillin 3 times a day and 500 mg of metronidazole 3 times a day. A course of treatment – 2 weeks, within the next 2 weeks – monotherapy by Zantak;
- Postoperative ulcers: on 150 mg 2 times a day, therapy duration – 4 weeks. In the absence of clinical effect treatment is prolonged for 4 weeks;
- Gastroesophageal reflux disease: on 150 mg: at an acute form – 2 times a day (or 1 time a day (for the night) in a dose of 300 mg), a course of treatment – 8-12 weeks; at a heavy and medium-weight current a reflux esophagitis – 4 times a day; for stopping of a pain syndrome – 2 times a day within 2-4 weeks; for prevention – 2 times a day;
- Zollingera-Ellison's syndrome: an initial dose – 150 mg 3 times a day, if necessary are possible increase in a daily dose to 6000 mg;
- Chronic incidental dyspepsia: on 150 mg 2 times a day, a course of treatment – 6 weeks. In the absence of clinical effect or an aggravation of symptoms carrying out additional inspection is necessary;
- Prevention of recurrent bleedings from round ulcers and bleedings of stressful ulcers at seriously ill patients of patients: after transfer of the patient into oral administration of food, parenteral therapy replace with Zantak's reception inside – 150 mg 2 times a day;
- Prevention of a syndrome of Mendelssohn: on 150 mg the day before (evening) and in 2 hours prior to the general anesthesia, perhaps parenteral use; during patrimonial activity – on 150 mg every 6 hours (in case of need simultaneous use of Zantak and water-soluble antacids is shown to the general anesthesia (sodium citrate)).
At a round ulcer at children purpose of drug is made taking into account the child's weight in a dose 2-4 mg on 1 kg 2 times a day, but no more than 300 mg a day.
At a heavy renal failure (the clearance of creatinine (CC) less than 50 ml/min.) plasma concentration of ranitidine therefore the dose has to make 150 mg of 1 times a day increases.
When finding the patient on a long hemodialysis or out-patient peritoneal dialysis drug appoint right after the end of a session in a dose 150 mg.
Solution for injections
Solution is intended for intermittent in/in infusion, intramuscular (in oil) or slow (more than 2 minutes) intravenous (in/in) an injection.
Slow in/in an injection it is appointed in a dose of 50 mg which is brought to volume of 20 ml and enter every 6-8 hours, an injection in oil – in a dose of 50 mg every 6-8 hours.
Intermittent it is necessary to carry out to infusion with a speed of 25 mg an hour within 2 hours with repetition of the procedure in 6-8 hours.
The recommended dosing:
- Prevention of a syndrome of Mendelssohn: slowly in/in or in oil – in a dose of 50 mg in 45-60 minutes prior to anesthesia;
- Prevention of a recurrence of bleeding from a round or stressful ulcer at seriously ill patients: begin with slow in/in an injection in a dose of 50 mg, then follows long in/in infusion with a speed of 0,125-0,250 mg at 1 kg of body weight an hour. Parenteral treatment lasts until independent meal of the patient, then oral administration of Zantak is possible;
- Treatment of patients with a renal failure (KK is less than 50 ml/min.): 25 mg.
Side effects
- Cardiovascular system: arrhythmia, lowering of arterial pressure (ABP), bradycardia, atrioventricular (AV) blockade; seldom – a vasculitis;
- Alimentary system: dryness in a mouth, nausea, vomiting, abdominal pains, a lock, reversible passing changes of tests of function of a liver; in some cases – development, usually reversible, cholestatic, hepatocellular or mixed hepatitis, with jaundice or without it; seldom – acute pancreatitis, diarrhea;
- System of a hemopoiesis: thrombocytopenia, leukopenia; seldom – a pancytopenia, an agranulocytosis; sometimes – immune hemolitic anemia, an aplasia and a hypoplasia of marrow;
- Musculoskeletal system: seldom – a mialgiya, an arthralgia;
- Nervous system: increased fatigue, headache (sometimes strong), drowsiness, dizziness; seldom – a sonitus, irritability, a sight illegibility, the involuntary movements, reversible motive disturbances of involuntary character; more often at elderly and seriously ill patients of patients – a hallucination, confusion of consciousness, a depression;
- Dermatological reactions: alopecia;
- Endocrine system: gynecomastia, giperprolaktinemiya, decrease in a libido, amenorrhea; seldom – swelling or a sensation of discomfort in chest glands at men, reversible impotence;
- Allergic reactions: urticaria, skin rash, bronchospasm, multiformny erythema, Quincke's disease, acute anaphylaxis, fever, arterial hypotension, thorax pains.
Special instructions
Zantak's use is capable to hide stomach carcinoma symptoms therefore patients with stomach ulcer or new symptoms of dyspepsia at advanced and middle age are recommended to exclude probability of a malignancy prior to treatment.
Because of risk of development of a syndrome of "ricochet" drug should be cancelled, gradually reducing a dose.
Long therapy of the weakened patients in the conditions of a stress can cause bacterial damage of a stomach with the subsequent intoxication of an organism.
The concomitant use of ranitidine with NPVS needs to be accompanied with regular observation of patients, especially at advanced age and at a peptic ulcer in the anamnesis.
It is necessary to take with care a sparkling pill to patients with sodium reception restriction, with a fenilketonuriya.
Zantak before diagnosis of skin tests on identification of allergic skin reaction of immediate type is not recommended to apply.
Patients with a heavy renal failure need correction of the mode of dosing.
Increase in level of hepatic transaminases can be observed at parenteral administration of high doses of drug more than 5 days.
Use of drug can cause increase in activity of a glutamattranspeptidaza or to become the reason of false positive reaction when carrying out the analysis to availability of protein in urine.
At tabakozavisimy patients efficiency of therapy by Zantak decreases.
During treatment the patient cannot take the products, drinks and medicines causing irritation of a mucous membrane of a stomach.
It is necessary to observe strictly recommended solution rate of administering.
Unused solution has to be destroyed within 24 hours.
During Zantak's use the patient cannot be engaged in potentially dangerous types of activity demanding concentration of attention and high speed of psychomotor reactions.
Medicinal interaction
Antacids, high doses of a sukralfat can break ranitidine absorption therefore at co-administration the interval between their reception and Zantak's reception has to be not less than 2 hours.
At a combination to oppressing marrow means the risk of a neutropenia increases.
Drug does not exert impact on effect of lidocaine, diazepam, Phenytoinum, theophylline, propranolol, warfarin and other means which are metabolized with the participation of isoenzymes of system of P450 cytochrome.
Drug suppresses metabolism of hexobarbital, Aminophenazonum, antagonists of calcium, phenazone, indirect anticoagulants, Buforminum, glipizid.
Not to allow decrease in absorption of a ketokonazol or itrakonazol, use of drug is shown only in 2 hours after their reception.
At simultaneous use with metoprololy ranitidine causes increase in its concentration in blood serum and increase in AUC.
Zantak for injections it is possible to mix from 0,9% or 0,18% solution of sodium of chloride, 5% or 4% solution of a dextrose, 4,2% sodium bicarbonate solution, Hartman's solution.
Terms and storage conditions
To protect from children.
To store at a temperature: tablets – to 30 °C, solution for injections – to 25 °C.
Period of validity: tablets with the engraving "GX EC2" – 5 years, "GX EC3" – 3 years, sparkling – 2 years; solution for injections – 3 years.
Sparkling to store tablets in tubas with densely closed cover.
Caries is the most widespread infectious disease in the world to which even flu cannot compete.

During foot walks blood moves on vessels more actively and one and all bodies are supplied with a large amount of oxygen. N...
Section: Slideshow
Striya (extension) are the defects of skin having an appearance of direct or wavy strips from 1 to 10 cm long and 1-5 mm wide. In most cases at women of a striya are located on a stomach, hips, a breast or buttocks. At athletes they can appear on shoulders and внутренн...
Section: Articles about health
They say that to ensure health and longevity of people it is obliged. Really, at competent approach to these questions, minimization of an adverse effect of many factors does not represent a special problem. Practically everyone has an opportunity to play sports, to pick up an optimum operating mode and rest, to adjust healthy food, to refuse addictions. It is more difficult to exclude hit in an organism of harmful substances through a respiratory organs: not all are able to afford to live in the area with хо...
Section: Articles about health
The concept "gluten" (differently, a gluten) combines group of the proteins which are a part of rye, barley and wheat. For most of people упот...
Section: Articles about health
The next flu epidemic leads to the next panic, from year to year we give in on these manipulations: professionally alarming voice of the announcer in news, reports with calculation of the died patients, an interview with people in white dressing gowns and advertizing of anti-influenza means ра...
Section: Articles about health
Many of us, probably, noticed more than once that from intellectual loadings at some point the brain as though "overheats" and "assimilation" of information is strongly slowed down. Especially this problem urgent for persons of age becomes more senior than fifty years. "Already badly I think", "the head will burst now", "memory as if is disconnected" - here that wants to be told at the time of information overload....
Section: Articles about health
The summer of this year in Russia was very ambiguous. Regions suffered from a merciless heat, from pouring rains, from times...
Section: Articles about health
The fatigue, sleep debt, disturbances of food, bad mood, vagaries of the weather – all these circumstances badly affect our appearance. Especially the person suffers: skin becomes flabby, loses healthy color, becomes covered by wrinkles, zones of hypostases and t appear...
Section: Articles about health
Women quite often suffer from complexes concerning the sizes of the bust. Strangely enough, not too modest, and excessively curvy shapes become the reason of sincere discomfort sometimes. Except psychological problems, the big bust sometimes creates also quite notable malfunctions with health: his owner can feel muscular dorsodynias, feeling of constant fatigue and difficulty of breath. Over time excess loading leads to development of diseases позвоночн...
Section: Articles about health
Helminthosis is one of the most widespread diseases. Statistically, with any species of helminths it is infected porridges...
Section: Articles about health
Frosty air, fresh wind and easy snowball at most of Russians are associated with cheerfulness, health and cheerful entertainments on which our winter is so generous. But, unfortunately, cold season sometimes brings also troubles with health. It is not about a season...
Section: Articles about health
The phenomenon of improvement of a condition of the patients at administration of drugs who are not containing active agents, so-called effect of placebo is known long ago. At the end of the 18th century the American doctor Perkins began to treat people the "miracle" sticks made of alloy of steel and brass. Was for several minutes to press such subject enough to a sore point that it became much easier for the patient. Having suspected Perkins of charlatanism, his colleagues tried to repeat "miracle" by means of sticks, steles...
Section: Articles about health
The state of health of the person in many respects depends on chemical composition of biological liquids of an organism. Specialists consider that з...
Section: Articles about health
Maternal milk is the best food for the newborn. It is the unique natural product containing an optimum set of nutrients, and which is best adapted in order that the baby normally developed and it was protected from harmful fa...
Section: Articles about health
History of cultivation of a buckwheat contains more than five thousand years. Grain which is received from this plant is used for preparation of porridges, soups, baked puddings and puddings, do flour which is one of the main ingredients of the noodles popular in many countries of it. Buckwheat dishes are useful and tasty, they are perfectly combined with meat, milk, eggs, mushrooms, fruit and vegetables....
Section: Articles about health
Residents of big cities quite often have a disease which is known as the syndrome of chronic fatigue (SCF) today. This illness...
Section: Articles about health
The winter swimming in open reservoirs called in our country by "winter swimming" – officially recognized sport and one of the most extreme ways of a hardening of an organism. This occupation has an old story and adherents in many countries. Are annually carried out...
Section: Articles about health
Within several decades of our compatriots convinced that the use of butter nasty affects a condition of coronary vessels. As a result the reputation of a product was impaired thoroughly a little, and many almost ceased to include it in the diet, having given preference "to safer" to vegetable fats. Meanwhile, the last researches showed that harm of butter for health is strongly exaggerated. But the product has a number of unique properties, to...
Section: Articles about health
Energy saving lamps are one of the most popular products of innovative technologies, and there is no wonder: they much эк...
Section: Articles about health
At this plant there are a lot of names: tuberiferous sunflower, Jerusalem artichoke, solar root, earth pear. Contrary to popular belief, it is not an exotic plant at all. The wild girasol grows in a midland of Russia practically everywhere: at the edges of roads...
Section: Articles about health
Today about 30 diseases, sexually transmitted are known. Wide circulation of these illnesses is extremely promoted by the dual attitude towards them: on the one hand, most of people know about "shameful" diseases very little and do not aim at receiving detailed and reliable information, considering that such problems personally will never concern them. With another – there are delusions about STD which instill unreasonable confidence that troubles such...
Section: Articles about health
Is told about advantage of domestic animals for development of the child much. But many parents nevertheless do not hurry to bring pets as about...
Section: Articles about health
Statistically, at the address to doctors seven of each ten patients complain of a headache. Actually it is much more people who are periodically feeling unpleasant feelings such. Many people, apart from a headache the reason for serious fear...
Section: Articles about health
All know that self-treatment is dangerous. However absolutely it is almost impossible to do without it. Rate of modern life does not allow to handle each small trouble to the doctor and information on ways of independent delivery of health care is quite available. Means, all of us have only one: to learn to give this help competently and in those limits in which it is possible for the person who does not have vocational education....
Section: Articles about health
About 10-15 years ago existence of the computer in the apartment of the Russian was considered as a rarity and office rooms were only on перв...
Section: Articles about health
People know that thermal sources have salutary force long ago. Treatment by natural waters is one of the most ancient methods of disposal of the most different diseases. Bathtubs, souls, wrappings and inhalations, in combination with reception of water vnut...
Section: Articles about health
Cellulitis - very widespread cosmetic shortcoming which arises approximately at 80% of women sooner or later. Emergence it is connected with change of structure of a hypodermic fatty layer. At the same time on the surface of skin at first there are roughnesses (cambers and cavities), and then small consolidations, the so-called effect of an orange-peel is shown. Changes in a condition of hypodermic cellulose are a consequence of a hormonal imbalance in an organism....
Section: Articles about health