Aklasta
Application instruction:
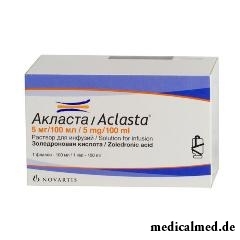 Aklasta – the drug inhibiting a bone resorption.
Aklasta – the drug inhibiting a bone resorption.
Form of release and structure
Dosage form of drug of Aklast – solution for infusions: transparent, colourless liquid (in polyethylene bottles on 100 ml, on 1 bottle in a cardboard pack).
Is a part of 1 bottle (100 ml):
- Active agent: zoledronovy acid (anhydrous) – 5 mg (monohydrate of zoledronovy acid – 5,33 mg);
- Auxiliary components: Mannitolum – 4950 mg; sodium citrate – 30 mg; water for injections – to 100 ml.
Indications to use
- Postmenopauzny osteoporosis (for the purpose of decrease in risk of development of fractures of vertebras, a femur and extra vertebral changes, and also for increase in mineral density of a bone);
- The osteoporosis caused by use of glucocorticosteroids (treatment and prevention);
- Postmenopauzny osteoporosis at women with an osteopeniya (prevention);
- Bone disease of Pedzhet;
- Prevention of new (subsequent) osteoporotichesky changes at women and men with changes of proximal department of a femur;
- Osteoporosis at men.
Contraindications
Absolute:
- Disturbances of mineral exchange in a heavy current, including a hypocalcemia;
- Functional disturbances of kidneys in a heavy current (at clearance of creatinine <35 ml/min.);
- Pregnancy and period of breastfeeding;
- Age up to 18 years (the profile of safety of Aklasta at this category of patients was not studied);
- Hypersensitivity to drug components, and also any bisfosfonata.
Relative, Aklasta is used with care in the presence of the following diseases / states:
- "Aspirinovy" bronchial asthma in the anamnesis;
- Functional disturbances of kidneys of average and easy severity;
- The accompanying oncological diseases and chemotherapy in the anamnesis;
- Heavy dehydration;
- The combined use with drugs which can exert significant impact on function of kidneys (for example, the antibiotics aminoglycosides or diuretic means causing dehydration).
Route of administration and dosage
Aklasta's solution is entered in the form of intravenous infusion, using for this purpose the valve infusional system providing constant rate of administering of solution for not less than 15 minutes.
Before performing infusion it is necessary to provide adequate hydration of an organism. In particular it is important for elderly patients (≥65 years), and also for the patients receiving diuretics.
The recommended scheme of use:
- Treatment of osteoporosis at men and postmenopauzny osteoporosis at women, and also the osteoporosis caused by use of glucocorticosteroids: 100 ml of solution (5 mg) once a year. In cases when receipt in an organism of vitamin D and calcium with food is not enough, in addition appoint the drugs covering this deficit;
- Prevention of the subsequent changes at patients with changes of proximal department of a femur: 100 ml of solution (5 mg) once a year. At recent (up to 3 months) changes of proximal department of a femur single use of high doses of vitamin D is recommended in 2 weeks prior to the first infusion (50000-125000 ME orally or intramusculary). At single introduction of Aklasta within 14 days before infusion by the patient it is recommended to accept daily inside calcium drugs (1000 mg a day) and vitamin D (800 ME in day). These drugs should be accepted within a year after infusion. The first infusion needs to be carried out in 2 and more weeks after operation;
- Prevention of postmenopauzny osteoporosis: 100 ml of solution (5 mg) of 1 times in 2 years. To make the decision on need of performing repeated infusion it is necessary to estimate annually risk of developing of fractures and the clinical response to therapy. In cases when receipt in an organism of vitamin D and calcium with food is not enough, to women in addition appoint the drugs covering this deficit;
- Treatment of a bone disease of Pedzhet: 100 ml of solution (5 mg) once. During the first 10 days after infusion the passing hypocalcemia can develop. Against the background of Aklasta's use reception of a sufficient dose of vitamin D and an adequate dose of calcium (not less than on 500 mg of elementary calcium 2 times a day) at least for the first 10 days after administration of drug is recommended;
- Repeated treatment of a bone disease of Pedzhet: repeated introduction of 100 ml of solution (5 mg) in 1 year or more from the beginning of therapy (duration of a break is defined individually on the basis of efficiency of drug and results of a research of level of an alkaline phosphatase (it is necessary to spend each 6-12 months). If clinical signs of an aggravation of symptoms (in the form of ostealgias and symptoms of a compression) and/or radiological signs of progressing of a disease are absent, the following infusion can be carried out not earlier, than in 12 months after the first.
At clearance of creatinine of ≥35 ml/min., functional disturbances of a liver and to elderly patients dose adjustment is not required.
Aklasta should not be mixed or entered together with any other medicines. It is impossible to allow contact with solutions, calciferous or any other bivalent cations.
For Aklasta's introduction it is always necessary to use separate system for infusions.
It is desirable to use solution right after opening of a bottle. If solution is cooled, before introduction he is recommended to be stood indoors before achievement of room temperature.
Side effects
The undesirable phenomena at treatment of osteoporosis at men, postmenopauzny osteoporosis at women, a bone disease of Pedzhet, prevention of the subsequent changes at patients with changes of proximal department of a femur, treatment and prevention of the osteoporosis connected using glucocorticosteroids, as a rule, carry poorly or moderately expressed character.
Development of disturbances lasting not longer 3 days in the form of fever, mialgiya, a grippopodobny syndrome, an arthralgia and a headache was most often noted. At repeated introduction of Aklasta expressiveness of side reactions considerably decreased.
Frequency of development of disturbances can be estimated on a scale: very often (≥1/10); often (≥1/100, <1/10); infrequently (≥1/1 000, <1/100); seldom (≥1/10 000, <1/1 000); very seldom (<1/10 000); with an unknown frequency (according to separate messages from clinical practice).
Disturbances, perhaps connected (according to attending physicians) with Aklasta's introduction at treatment of a bone disease of Pedzhet, different types of osteoporosis, prevention of new changes at patients with changes of proximal department of a femur:
- Alimentary system: often – vomiting, nausea, diarrhea; infrequently – pain in upper parts of a stomach, dyspepsia, anorexia, a loss of appetite, an abdominal pain, an esophagitis, dryness in a mouth, a gastroesophageal reflux, a lock, a dentagra, gastritis (against the background of therapy by glucocorticosteroids);
- Skeletal and muscular system and connecting fabric: often – mialgiya, arthralgias, an ostealgia, extremities and a back; infrequently – pain in a neck, muscular spasms, swelling in joints, pains in the field of a shoulder girdle and a thorax (a musculoskeletal origin), weakness in muscles, constraint in joints and muscles, musculoskeletal pains, arthritises; with an unknown frequency – a jaw osteonecrosis;
- Urinary system: infrequently – a pollakiuria, increase in level of creatinine of blood, a proteinuria; with an unknown frequency – a renal failure;
- Respiratory system: infrequently – cough, short wind;
- Nervous system: often – a headache, dizziness; infrequently – block, a syncope, paresthesias, a tremor, drowsiness, a dysgeusia;
- Hemopoietic system: infrequently – anemia;
- Cardiovascular system: often – fibrillation of auricles; infrequently – sudden face reddening, increase in arterial pressure, a heart consciousness; with an unknown frequency – the expressed lowering of arterial pressure (with risk factors);
- Hypodermic cellulose and skin: infrequently – rash, an itch, a hyperhidrosis, an erythema;
- Sense bodys: often – a sclera hyperemia; infrequently – eye pain, conjunctivitis, вертиго; seldom – a uveitis, an episcleritis, an iritis; with an unknown frequency – an inflammation of an eye-socket and a sclera;
- Invasions and infections: infrequently – flu, a nasopharyngitis;
- Mentality: infrequently – sleeplessness;
- Organism in general and disturbances in an injection site: very often – fervescence; often – reactions in the place of infusion, a fever, a grippopodobny syndrome, increased fatigue, pain, an adynamy, a febricula; infrequently – feeling of thirst, peripheral hypostases, ostrofazny reactions, the stethalgia (which is not connected with heart troubles); with an unknown frequency – dehydration (arises for the second time in relation to post-infusional to symptoms, such as vomiting, fever and diarrhea).
When carrying out separate researches the following undesirable phenomena which emergence frequency in Aklasta's group was lower, than at the patients who were not receiving drug were recorded: a hypocalcemia, reactions in an injection site, reddening of eyes, gastritis, increase in content of S-reactive protein, a dentagra, a dysgeusia, feeling of heartbeat.
At Aklasta's use for women with postmenopauzny osteoporosis development of fibrillation of auricles was noted (this disturbance is not confirmed with other clinical trials of zoledronovy acid).
The general profile of safety at Aklasta's use at postmenopauzny osteoporosis (with the preventive purpose) is comparable with that at treatment of postmenopauzny osteoporosis, except the side reactions which are observed within 3 days after infusion (in the form of pain, fever, a fever, a mialgiya, nausea, a headache, increased fatigue, an arthralgia) which frequency was higher at the patients using drug for prevention. Most often these side reactions were easy or average degree of manifestation and took place independently within 3 days after emergence. At repeated use of Aklasta expressiveness of these undesirable phenomena considerably decreased.
Side reactions, perhaps connected using Aklasta for prevention of postmenopauzny osteoporosis (according to attending physicians):
- Alimentary system: very often – nausea; often – anorexia, a lock, an abdominal pain and upper parts of a stomach;
- Nervous system: very often – a headache; often – block, a tremor; infrequently – a dysgeusia, a hyposensitivity;
- Skeletal and muscular system and connecting fabric: very often – a mialgiya; often – pain in a thorax of a musculoskeletal origin, a spasm of muscles, pain in a jaw and a neck; infrequently – a stitch;
- Mentality: infrequently – alarm;
- Skin and hypodermic cellulose: often – the increased sweating at night;
- Organ of sight: often – eye pain, conjunctivitis, an iritis; infrequently – indistinct sight;
- Organism in general and disturbances in an injection site: very often – a fever, pain; often – reactions in a solution injection site, peripheral hypostases, not cardial pain in a thorax.
At postmenopauzny osteoporosis against the background of performing therapy cases of decrease in concentration of calcium in blood serum without clinical signs of a hypocalcemia were noted. At treatment of a bone disease of Pedzhet approximately in 1% of cases development of the passing hypocalcemia which is followed by clinical manifestations was noted.
During use of zoledronovy acid cases of renal failures with increase in concentration of creatinine of blood which sometimes were followed by an acute renal failure were noted (it is necessary to be careful at Aklasta's use for patients with the accompanying oncological diseases and chemotherapy).
Cases of emergence of an osteonecrosis (in the majority of episodes – jaws) generally took place at oncological patients after extraction of tooth or other dental manipulations. As a rule, they observed symptoms of local infectious and inflammatory process, including osteomyelitis.
Also during therapy the following undesirable phenomena were noted (relationship of cause and effect using Aklasta is not established): hypersensitivity reactions, including in rare instances urticaria, a bronkhokonstriktion, a Quincke's disease, anaphylactic reactions / shock.
In some cases in clinical practice development of functional disturbances of kidneys, including a renal failure at which carrying out a hemodialysis, or cases of a lethal outcome, in particular was required from patients with existence of accessory factors of risk (for example, the accompanying therapy by diuretics and nephrotoxic drugs, advanced age or heavy dehydration) or renal pathology in the anamnesis was noted.
Special instructions
Patients need to know the main manifestations of a hypocalcemia. Behind a condition of the patients entering into risk group regular medical observation has to be established.
To patients with a bone disease of Pedzhet only qualified specialists with experience of treatment of this disease have to appoint therapy.
To reduce the frequency of development of the side reactions arising within 3 days after Aklasta's introduction use of paracetamol or an ibuprofen shortly after infusion is possible.
At a hypocalcemia prior to use of drug it is necessary to carry out treatment by adequate doses of calcium and vitamin D. At other disturbances of mineral exchange (for example, the operations which developed after carrying out on parathyroid and thyroid glands, at a hypoparathyrosis or decrease in absorption of calcium in intestines) it is also necessary to carry out therapy.
To reduce probability of emergence of renal failures, it is necessary to observe the following instructions:
- Aklast should not appoint the patient with heavy renal failures (because of limited data on safety of performing therapy at this category of patients);
- At the combined use it is necessary to be careful with drugs/substances which can exert significant impact on function of kidneys;
- Before Aklasta's introduction it is necessary to define clearance of creatinine. At use of drug for patients who have risk factors of development of renal disturbances it is necessary to determine the content of creatinine in serum regularly;
- Before administration of solution it is necessary to provide adequate hydration of an organism, in particular to patients 65 years, and also the patient receiving therapy by diuretics are more senior;
- The single dose should not exceed 5 mg. The drug should be administered for not less than 15 minutes.
Oncological diseases, the accompanying therapy (for example, radiation therapy, chemotherapy, treatment by glucocorticosteroids) and existence of other accompanying diseases belong to risk factors of emergence of an osteonecrosis (for example, infections, anemia, coagulopathies, diseases of teeth in the anamnesis). Before Aklasta's introduction it is necessary to conduct dental examination and, with risk factors, to perform necessary preventive procedures in advance.
Patients have data on cases of atypical diaphyseal and subtrochanterian fractures of femur, is long receiving bisfosfonata. These changes, as a rule, develop spontaneously or after an insignificant injury and can be followed by bad accretion. The doctor to accept the decision on cancellation of use of Aklasta for patients with suspicion of atypical fractures of a femur individually on the basis of assessment of a ratio of advantage and risk.
It is necessary to be careful when performing potentially dangerous types of works as during use of drug development of dizzinesses is possible.
Medicinal interaction
Special researches on studying of interaction of zoledronovy acid with other medicines were not conducted.
It is necessary to be careful at the combined use of Aklasta with the medicinal means / substances which are significantly influencing function of kidneys.
At functional disturbances of kidneys Aklasta's combination with drugs which are removed preferential by kidneys can lead to increase in systemic action of these medicines.
Aklasta's solution is incompatible with solutions which contain calcium or other bivalent cations (for example, in one system for intravenously drop introduction).
Terms and storage conditions
To store in the place, unavailable to children, at a temperature up to 25 °C.
Period of validity – 3 years.
Solution after opening of a bottle keeps stability for 24 hours at its storage at a temperature of 2-8 °C.
Name of drug
Price
Drugstore
Aklasta solution for инф 5 mg фл 100 ml, Novartis Pharma
18011 rub.
 Network of the Moscow drugstores of IFC
Network of the Moscow drugstores of IFCThe person accepting antidepressants in most cases will have a depression again. If the person coped with depression by own efforts, he has every chance forever to forget about this state.

Bathing in broths of medical flowers and plants (phytobathtub) was eurysynusic since Cleopatra who is a good judge of everything...
Section: Articles about health
Eyes – unique body on the structure thanks to which the person obtains about 80% of information on the world around: about a form, color, size, the movement, and also many other parameters of objects or phenomena. But whether much we know about the most valuable body...
Section: Articles about health
Venereal diseases in medicine are called the infections which are transmitted preferential sexually, now they and are called - infections, sexually transmitted, or STD. Among them is also life-threatening. In spite of the fact that the majority of diseases such will respond to treatment, they are widespread everywhere, and there is no tendency to decrease in incidence. Besides, some of them promptly look younger: statistically, a third of young people at the age of 16-22 years of a str...
Section: Articles about health
About 10-15 years ago existence of the computer in the apartment of the Russian was considered as a rarity and office rooms were only on перв...
Section: Articles about health
It is impossible to imagine human life in which there would be no plants. Practically in each apartment and any production room there are window plants, millions of people with pleasure are engaged in gardening and truck farming, many citizens пр...
Section: Articles about health
Water with a lemon - idle time in preparation drink which supporters of a healthy lifestyle already managed to appreciate. Used in a warm look and on an empty stomach, it is one of the most useful prophylactics allowing to prevent tens of diseases and just to raise an organism tone. Especially effectively to use warm water with lemon juice after a serious illness, during a season of the colds, and also to children, old men and pregnant women which do not have contraindications...
Section: Articles about health
Scientists always aimed to offer fundamental explanations for medical problems. Their theories formed the basis of modern methods is treated...
Section: Articles about health
Separate food - the system of meal based on digestion physiology which is carried to improvement methods. According to nutritionists, the separate use of the carbohydrate and proteinaceous products demanding different conditions of assimilation helps to get rid from Bol...
Section: Articles about health
Visit of doctors – business not the most pleasant, and many people do not hurry to undergo necessary planned inspections. Such behavior is extremely thoughtless and improvident. Our health is necessary not only to us: wellbeing of darlings, children, grandsons and aged parents directly depends on as far as we are vigorous and able-bodied. Therefore in time to be inspected – a duty of any modern person. Specialists consider that 7 regular surveys and di are especially necessary for women...
Section: Articles about health
Scientists have no unambiguous opinion on a proximate cause of emergence of a carcinoma cutaneum today. Are precisely established only фа...
Section: Articles about health
The endocrine system carries out extremely important role in a human body, practically all processes of life activity are regulated by it. Closed glands (hemadens) produce special biologically active agents – hormones which then o...
Section: Articles about health
Transfusion of donor blood has almost century history. In spite of the fact that this procedure is quite usual for many people, process of blood donation is still surrounded with numerous myths. Today we aimed to discredit the most widespread of them....
Section: Articles about health
Olive oil – the product capable to make a powerful contribution to health of the person if it includes it in the diet. Rich vitamin...
Section: Articles about health
The name of this disease precisely reflects the problem reason: it consists in the bra fastener pressure upon a certain zone of a back. At the same time one of vertebrae of chest department of a backbone is as if blocked and loses mobility, and falling on it is nude...
Section: Articles about health
Smack in a mouth can arise in the natural way – as a result of lack of morning hygiene or reception of the corresponding food. However in certain cases its existence is a sign of certain pathologies, and allows to reveal an illness at an early stage. Depending on character of aftertaste – acid, salty, bitter, sweet – distinguish also diseases which accompany it....
Section: Articles about health
It would seem, to buy drugs in Moscow does not make a problem – a drugstore, and not one, is available for each resident of the capital in step a toast...
Section: Articles about health
For the time being the perspective of heart diseases seems to most of people remote and foggy. But sooner or later practically each adult faces extremely unpleasant feelings: sudden stethalgia. To be consoled at this time in a thought of t...
Section: Articles about health
Physical activity is necessary for normal functioning of a human body. At a lack of the movement joints cease to function, muscles atrophy, cardiovascular activity is broken and the metabolism worsens. The modern city rhythm of life does not provide the person with an adequate exercise stress, additional - sport is necessary. Tedious tasks the huge number of the people having those or ин exists sport not all to liking, but also...
Section: Articles about health
One of the major chemical processes happening in a human body are oxidation reactions. They go with participation of fats...
Section: Articles about health
Several decades ago the basil (the district khan, реан, Reagan) was considered as a part of the Caucasian or east cuisine, but today it strongly took the place on tables of Russians. Greens of this plant possess a strong, pleasant smell and specific fresh taste, because of to...
Section: Articles about health
The depression not without reason is considered one their main troubles of our century: for scientific and technical progress, acceleration of rate of life and a surplus of information of people it is forced to pay with stresses, negative emotions and weakening of protective forces of an organism. As a result widely the states which are characterized by the increased uneasiness, falling of interest in life, spiritual and physical discomfort extend....
Section: Articles about health
Doctors claim that the people not so familiar with a dorsodynia occur among adult Russians very seldom. At the same time подавляющ...
Section: Articles about health
People know that thermal sources have salutary force long ago. Treatment by natural waters is one of the most ancient methods of disposal of the most different diseases. Bathtubs, souls, wrappings and inhalations, in combination with reception of water vnut...
Section: Articles about health
Statistically, pathologies of a thyroid gland in the world more than 500 million people have. Failures in work of this body lead to heavy disbolism, development of heart diseases, vessels, a reproductive and nervous system. In hard cases excess or insufficient production of the main hormones of a thyroid gland (thyroxine and triiodothyronine) leads to essential decline in quality of life and disability....
Section: Articles about health
The mankind knows that some toxins at intake in the minimum quantities have therapeutic effect...
Section: Articles about health
Cold, puffiness of a nose, itch, the watering eyes - characteristic symptoms of the allergic rhinitis resulting from hit of allergens (pollen, house dust, hair of animals, etc.) on a mucous membrane of a nose. Unpleasant feelings often deliver беспоко...
Section: Articles about health
With age in a human body harmful substances collect. We receive them with food and water, at inhalation of the contaminated air, reception of medicines, use of household chemicals and cosmetics. A considerable part of toxins accumulates in a liver which main function is continuous purification of blood. This body begins to knock as any got littered filter, and efficiency of its work decreases....
Section: Articles about health