Dipiridamolum
Application instruction:
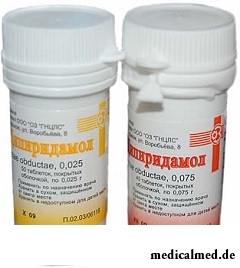 Dipiridamolum – vazodilatiruyushchy drug.
Dipiridamolum – vazodilatiruyushchy drug.
Form of release and structure
Dosage forms:
- Tablets, coated (on 10 pieces in a blister strip packaging, in a cardboard pack: on 25 and 50 mg – 5 or 10 packagings, on 75 mg – 4 or 10 packagings);
- Suspension for intake: bright yellow liquid with a characteristic smell of almonds (on 150 ml in bottles from orange glass (type III), in a cardboard pack 1 bottle).
Active agent – Dipiridamolum:
- 1 tablet – 25, 50 or 75 mg;
- 5 ml of suspension – 50 mg.
Suspension excipients: ammonium glycyrrhizinate, magnesium aluminum silicate, propylene glycol (E1520), polysorbate 80, мальтитол liquid (E965), sodium hydroortho-phosphate anhydrous (E339), citric acid of monohydrate solution of 5%, an emulsion of a simetikon of 30% (Q7-2587), left menthol, methylparahydroxybenzoate (E218), sodium of hydroortho-phosphate anhydrous solution of 5%, пропилпарагидроксибензоат (E216), gum xanthane (E415), fragrance Almonds (F31209) (propylene glycol and ethanol are a part), citric acid monohydrate (E330), the water purified.
Indications to use
- Prevention and treatment of disturbances of cerebral circulation on ischemic type;
- Distsirkulyatorny encephalopathy;
- Prevention of venous and arterial thromboses and their complications;
- Prevention of a thromboembolism after surgical intervention on prosthetics of valves of heart;
- As a part of complex treatment of all types of disturbance of microcirculation;
- Prevention and treatment of the acute respiratory viral infections (ARVI), flu, as an immunomodulator and the inductor of interferon (only tablets).
Besides, tablets of Dipiridamolum apply at the complicated pregnancy to prevention of placental insufficiency.
Contraindications
- The acute myocardial infarction, unstable stenocardia widespread the stenosing atherosclerosis of coronary vessels;
- Dekompensirovanny heart failure;
- Subaortal stenosis;
- The expressed arterial hypotension;
- Heavy disturbances of a cordial rhythm;
- The expressed renal failure;
- Tendency to bleedings;
- Hypersensitivity to Dipiridamolum and auxiliary components of drug.
Contraindications to suspension use also are:
- Liver failure;
- Heavy arterial hypertension;
- Hypertrophic subaortic stenosis;
- Hemorrhagic diathesis;
- Chronic obstructive pulmonary disease;
- Diseases with tendency to bleedings, including stomach ulcer and a duodenum;
- Age up to 18 years.
Dipiridamolum should be taken with caution:
- Tablets: the patient with recently postponed myocardial infarction; arterial hypotension; heart failure; at pregnancy, in urgent cases, especially in the II-III trimesters;
- Suspension: diseases of coronary arteries, including a state after a myocardial infarction and/or unstable stenocardia; disturbances of coagulability of blood; during pregnancy and breastfeeding, only in cases when by definition of the doctor the expected effect for mother exceeds potential risk for a fruit and the child (drug cosecretes in breast milk).
Route of administration and dosage
- Tablets: accept inside. The daily dose can fluctuate from 50 to 600 mg, frequency rate of reception and the period of treatment are appointed by the doctor on the basis of clinical indications;
- Suspension: accept inside in 1 hour prior to food. Before each reception it is recommended to shake up bottle contents. Dosing is appointed by the attending physician individually depending on clinical indications, severity of a condition of the patient and his reaction to treatment. The recommended daily dosing: for reduction of aggregation of thrombocytes – 300 mg: on 100 mg (2 teaspoons) 3 times, for hard cases are allowed increase in a daily dose up to 600 mg; prevention of flu and other SARS (it is most urgent during the epidemiological period) – 50 mg (1 teaspoon) once a week, course duration – 4-5 weeks; prevention of a recurrence at the patients who often have viral respiratory infections – 100 mg (2 teaspoons) 2 times a day once a week, duration – 8-10 weeks.
Side effects
Undesirable effects of reception of tablets can become:
- Cardiovascular system: at reception of high doses – inflows, arterial hypotension, tachycardia, are more often at a combination to vazodilatator;
- Alimentary system: nausea, vomiting, diarrhea;
- Nervous system: headache, dizziness;
- Allergic reactions: urticaria, skin rash.
Reception of suspension of Dipiridamolum can cause side effects:
- From blood and system of a homeostasis: infrequently – thrombocytopenia, disturbance of functional properties of thrombocytes; seldom – bleedings; very seldom – the raised bleeding;
- From the alimentary system: infrequently – pain in epigastric area, diarrhea, nausea, vomiting;
- From cardiovascular system: infrequently – rushes of blood to the person, a heart consciousness, bradycardia, tachycardia, a lowering of arterial pressure, a syndrome of coronary burglarizing (at a dose more than 225 mg a day);
- Others: sonitus, weakness, dermahemia of the person, dizziness, headache, feeling of a congestion of an ear, rhinitis, arthritis, allergic reactions (urticaria, rash, Quincke's disease, strong bronchospasm).
Usually, use of suspension in therapeutic doses does not cause the expressed side effects, as a rule, they are temporary.
In rare instances reception of tablets and suspension can cause plentiful bleeding in time or after carrying out surgical intervention and a mialgiya.
At emergence of these or other side effects at use of Dipiridamolum it is necessary to consult with the doctor.
Special instructions
Intravenous administration of Dipiridamolum as there is a risk of development of a syndrome of burglarizing with aggravation of symptoms of stenocardia is not recommended.
At complex therapy of patients with a severe form of a myasthenia, carrying out correction of doses of drugs against the background of reception of Dipiridamolum is necessary.
At constant administration of drug inside, it is not necessary to appoint additional intravenous administration of Dipiridamolum.
In case of need test of loading for diagnosis of diseases of coronary arteries, administration of drug inside should be cancelled in 24 hours prior to its intravenous administration.
Drug can cause side effects in the form of weakness, dizziness and a lowering of arterial pressure therefore during treatment patients need to be careful at all types of activity demanding the high speed of psychomotor reactions, concentration of attention including at control of vehicles.
Medicinal interaction
At simultaneous use of each of dosage forms of Dipiridamolum:
- Anticoagulants (heparin, trombolitik), acetylsalicylic acid – antiagregantny action of tablets and suspension amplifies, the risk of development of hemorrhagic complications increases;
- The drugs reducing arterial pressure – their hypotensive activity increases;
- Antacids, blockers histamine H2 receptors (Cimetidinum), inhibitors of the protonew pump, xanthine derivatives (theophylline, caffeine) – decrease efficiency of Dipiridamolum.
At a concomitant use of Dipiridamolum in the form of tablets:
- Anticholinergics – can cause disturbances of attention and memory in elderly patients;
- Beta adrenoblockers – can cause bradycardia and an asystolia;
- Adenosine – action of adenosine amplifies;
- Dobutaminum – raises risk of heavy arterial hypotension.
At Dipiridamolum suspension combination:
- With cholinesterase inhibitors – decrease in their anticholinergic properties is possible;
- With antibiotics of a tsefalosporinovy row (цефамандол, цефотетан, цефоперазон) – action of Dipiridamolum amplifies.
Terms and storage conditions
To store at a temperature up to 25 °C, tablets – in the dry place. To protect from children.
Period of validity: tablets – 5 years, suspension – 2 years.
According to opinion of many scientists, vitamin complexes are almost useless for the person.
Scientists have no unambiguous opinion on a proximate cause of emergence of a carcinoma cutaneum today. Are precisely established only фа...
Section: Articles about health
All know that self-treatment is dangerous. However absolutely it is almost impossible to do without it. Rate of modern life does not allow to handle each small trouble to the doctor and information on ways of independent rendering medical the help...
Section: Articles about health
Since the moment when the child becomes a school student, his sight begins to be exposed to the strengthened loadings which are supplemented with viewing of animated films and long computer games. During this period of life of the child development of not completely created organs of sight, it is very easy to break the excessive loading which is aggravated with lack of a work-rest schedule. As a rule, and occurs: according to WHO statistics, every fourth child of school age has these or those diseases of eyes, Wednesday...
Section: Articles about health
Olive oil – the product capable to make a powerful contribution to health of the person if it includes it in the diet. Rich vitamin...
Section: Articles about health
Almost each of us during life faced dissatisfaction with own body. At such moments, as a rule, we begin to shame ourselves, urgently we go on the most rigid diet promising minus of 10 kg in a week, or we exhaust ourselves in the gym to полусм...
Section: Articles about health
Hemorrhoids – extremely widespread disease. Periodically arising inflammations and bleeding of hemorrhoidal nodes cause serious discomfort to nearly fifteen percent of adults. Meanwhile, having a clear idea of the reasons of an exacerbation of an illness and following rules of precaution, it is possible to reduce substantially sharpness of unpleasant feelings and to reduce progressing of a disease....
Section: Articles about health
People know that thermal sources have salutary force long ago. Treatment by natural waters is one and...
Section: Articles about health
Statistically, at the address to doctors seven of each ten patients complain of a headache. Actually it is much more people who are periodically feeling unpleasant feelings such. Many people, apart from a headache the reason for serious fear...
Section: Articles about health
Extracorporal fertilization – one of the most modern methods of controlling with infertility. So far he already helped a significant amount of married couples to become happy parents. Usually to the EKO procedure difficult and very expensive, resort in those situations when all other ways to help couple to bring the child are inefficient. "Conception in a test tube" yields quite good results in cases of infertility of one of partners, existence at the woman of impassability of uterine tubes...
Section: Articles about health
Striya (extension) are the defects of skin having an appearance of direct or wavy strips from 1 to 10 cm long and 1-5 mm wide. In the majority with...
Section: Articles about health
The nature does not stand stagnation and monotony. It is known that tissues of a human body atrophy if do not receive necessary loadings. It fully belongs also to a cerebral cortex: when it is not given full-time job, it begins to function worse. In резуль...
Section: Articles about health
80% of women at least once to lives complained of discomfortable feelings to breasts, consolidations and nagrubaniye. These are mastopathy symptoms. The mastopathy is characterized by change of a ratio between ferruterous and connective tissue tissues of mammary glands. It can lead to formation of cysts (a cystous mastopathy), gland consolidation (a fibrous mastopathy), or a combination of these processes (a fibrous and cystous mastopathy)....
Section: Articles about health
The winter swimming in open reservoirs called in our country by "winter swimming" – officially recognized sport and one of the ek...
Section: Articles about health
Healthy lifestyle today in fashion, and many parents think of that the child from the early childhood played sports. Trainings will help it to become strong and hardy, will improve coordination of movements, and also will exert positive impact on mentality: it...
Section: Articles about health
Cold is such painful that each sigh becomes a victory, heat "knocks" down, and the ache in joints forces to think only of pain. Some people with approach of the first symptoms of cold make the self-sacrificing decision to have a disease standing, and at best to rest in bed with a cup of hot tea. There is an opinion that if not to treat cold, then the organism itself, sooner or later, will overcome an illness. Whether so it? It is known that if in time it is simple not to begin treatment, apparently, harmless...
Section: Articles about health
Statistically, can only one of ten of our compatriots brag of a decent condition of an oral cavity. On среднестатистич...
Section: Articles about health
It is possible to find the extensive range of fruit and vegetables in modern shops. Russians already got used that on counters there is not only a seasonal domestic production, but the vegetables and fruit which are grown up in the countries with more comfortable conditions at all seasons of the year...
Section: Articles about health
Cold – a state known to everyone which is followed by cold, cough, high temperature, a pharyngalgia. Often the first that we begin to do in hope again to become healthy – to accept medicines which are not always harmless whereas it is easy to facilitate displays of a disease by means of natural means. They not only softly eliminate disease symptoms, but also enrich the weakened organism with useful substances. We present you 8 drinks which are successfully used for...
Section: Articles about health
We live during an advertizing era. Daily each person receives a solid portion of persuasive councils about what to eat to be здо...
Section: Articles about health
Radiological methods of a research are applied in medicine more than hundred years, and thanks to them millions of lives were saved. In many cases without X-ray it is impossible to make exact idea of a condition of bodies and fabrics, it is correct to make the diagnosis. Those...
Section: Articles about health
Several decades ago the basil (the district khan, реан, Reagan) was considered as a part of the Caucasian or east cuisine, but today it strongly took the place on tables of Russians. Greens of this plant possess a strong, pleasant smell and specific fresh taste because of which it is included almost in all dry mixes of spicy herbs, and also give to meat and fresh fish dishes....
Section: Articles about health
Dark circles (bruises) under eyes – a shortcoming with most of which often fight against the help of cosmetics (proofreaders, salons...
Section: Articles about health
Ayurveda - the most ancient tselitelsky practice which came to us from India. It represents the doctrine about maintenance of physical, psychological and moral health of the person by means of the complex of procedures including a diet, cleaning of an organism, respiratory упр...
Section: Articles about health
They say that to ensure health and longevity of people it is obliged. Really, at competent approach to these questions, minimization of an adverse effect of many factors does not represent a special problem. Practically everyone has an opportunity to play sports, to pick up an optimum operating mode and rest, to adjust healthy food, to refuse addictions. It is more difficult to exclude hit in an organism of harmful substances through a respiratory organs: not all are able to afford to live in the area with хо...
Section: Articles about health
Each woman has preferences in the field of use of those goods which help us to look good, feel се...
Section: Articles about health
Eyes – unique body on the structure thanks to which the person obtains about 80% of information on the world around: about a form, color, size, the movement, and also many other parameters of objects or phenomena. But whether much we know about the most valuable body...
Section: Articles about health
Summer in the heat. Many are going to spend vacation abroad. Travelers the tender seas, rest on beaches wait, for sightseeing, campaigns on natural and cultural reserves. But, unfortunately, on vacation also problems with health can wait for us. On a foreign trip it is possible to face also diseases which not only will spoil long-awaited issue, but also will force to be treated within long months after its termination. To be insured completely from troubles of it a sort...
Section: Articles about health