Kanamycinum
Application instruction:
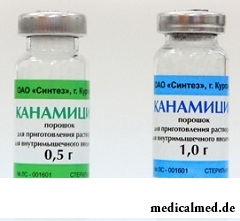 Kanamycinum – antibiotic drug of group of aminoglycosides.
Kanamycinum – antibiotic drug of group of aminoglycosides.
Form of release and structure
Kanamycinum is released in the following dosage forms:
- Powder for preparation of solution for intravenous and intramuscular administration (in bottles on 10 ml, on 1, 10, 50 bottles in a cardboard pack);
- Powder for preparation of solution for intramuscular introduction (in bottles on 10 ml, on 1, 5, 10, 50 bottles in a cardboard pack);
- Powder for preparation of solution for injections (in bottles, on 1, 10, 50 bottles in a cardboard pack).
Active agent is a part of 1 bottle: Kanamycinum – 500 or 1000 mg (in the form of monosulphate).
Indications to use
- Infectious and inflammatory diseases of a respiratory organs, including pneumonia, lung abscess, a pleura empyema;
- Heavy it is purulent - septic diseases, including peritonitis, meningitis, sepsis, a septic endocarditis;
- The purulent complications developing in the postoperative period;
- Infections of urinary tract and kidneys, including cystitis, pyelonephritis, an urethritis;
- The pulmonary tuberculosis and tubercular diseases of other bodies caused by the tuberculosis mycobacteria showing stability to antitubercular medicines I and II of a row and other antitubercular drugs except a florimitsin;
- The infected burns and other diseases caused by preferential gram-negative microorganisms (Klebsiella pneumonia, E. coli, Serratia, Enterobacter aerogenes, Salmonella, Proteus spp., Shigella), showing other antibiotic drugs resistance, or associations of gram-negative and gram-positive activators.
Contraindications
- Neuritis of the VIII couple of cranial nerves;
- Heavy chronic renal failure with an azotemia and uraemia;
- Pregnancy;
- Hypersensitivity to drug components (including to other aminoglycosides in the anamnesis).
The feeding women should appoint Kanamycinum with care, to elderly patients, premature children and children is younger than 1 month, and also the patient with a myasthenia, parkinsonism, a renal failure and botulism (aminoglycosides can lead to neuromuscular transmission disturbance that will cause further weakening of skeletal muscles).
Route of administration and dosage
Kanamycinum is applied intramusculary, intravenously (kapelno), in-band.
At intravenous administration (kapelno) a single dose (500 mg) it is necessary to dissolve 5% of solution of a dextrose in 200 ml. The drug is administered with a speed of 60-80 thaws a minute.
At treatment of infections of not tubercular etiology the adult single dose makes 500 mg, daily – 1000-1500 mg (each 8-12 hours on 500 mg). The highest daily dose – 2000 mg. Duration of a therapeutic course – 5-7 days. To children Kanamycinum is entered only intramusculary on 50 mg/kg a day. Premature and to children of the first month of life drug is appointed only according to vital indications.
At treatment of tuberculosis Kanamycinum is applied intramusculary. The mode of dosing is defined by age:
- Adults: once a day on 1000 mg or 2 times a day on 500 mg;
- Children: on 15-20 mg/kg a day (as much as possible – 500-750 mg a day).
Every seventh day of treatment should be done a break.
Into cavities (pleural, belly, joint) Kanamycinum is entered for washings. A single dose – 10-50 ml of 0,25% of aqueous solution.
Intraperitoneally enter 2,5% of solution on 500 mg.
When carrying out peritoneal dialysis it is necessary to dissolve 1000-2000 mg of Kanamycinum in 500 ml of the dialyzing liquid.
In the form of heatwet inhalations and an aerosol (at a temperature of solution of 35-40 °C) Kanamycinum is applied at treatment of infections of respiratory tract of not tubercular etiology and a pulmonary tuberculosis. For this purpose 250-500 mg of drug should be dissolved in 3-5 ml of the distilled water or 0,9% of solution of sodium chloride. Frequency rate of introduction – 2 times a day. Apply the following mode of dosing (a single/daily dose):
- Adults: 500/500-1000 mg;
- Children: 5/15 mg/kg.
Duration of therapy is defined by indications: acute diseases – 7 days, chronic pneumonia of-15-20 days, a pulmonary tuberculosis – 30 days and more.
The scheme of introduction for patients with a renal failure should be adjusted by decrease in doses or increase in breaks between introductions (it is recommended to apply the following formula to calculation: a break between introductions in hours = the content of creatinine in a blood plasma (mg / 100 ml) × 9).
The initial dose of Kanamycinum is calculated taking into account body weight (a dose, mg = body weight × 7). For calculation of the subsequent doses the initial dose needs to be divided into the content of creatinine in blood serum (mg / 100 ml).
Frequency rate of use – 2-3 times a day. In days of a hemodialysis after its carrying out it is necessary to enter an additional single dose of drug.
To avoid overdose, it is recommended to control periodically concentration of Kanamycinum in the patient's blood.
Side effects
During use of Kanamycinum development of the following side effects is possible:
- Urinary system: nephrotoxicity – functional disturbances of kidneys (thirst, increase or decrease in frequency of an urination, a microhematuria, a cylindruria, an albuminuria);
- Nervous system: weakness, drowsiness, a headache, neurotoxic disturbances (epileptic seizures, feeling of a pricking and numbness, twitching of muscles, paresthesias), disturbance of neuromuscular transfer is possible;
- Digestive tract: functional disturbances of a liver (hyperbilirubinemia, increase in activity of hepatic transaminases), nausea, diarrhea, vomiting;
- Sense bodys: ototoxicity (feeling of a mortgaging or a ring in ears, a hearing impairment up to irreversible deafness), toxic impact on a vestibular mechanism (dizziness, a diskoordination of movements, vomiting and nausea);
- Bodies of a hemopoiesis: thrombocytopenia, leukopenia, anemia, granulocytopenia;
- Allergic reactions: itch, skin rash, fever, dermahemia, Quincke's edema.
Special instructions
During use of Kanamycinum follows at least 1 time in 7 days to control function of a vestibular mechanism, acoustical nerve and kidneys.
The probability of development of nephrotoxicity is higher at patients with functional disturbances of kidneys, and also at use of high doses of drug or at long therapy (such patient daily control of function of kidneys can be required).
At unsatisfactory results of audiometric tests it is necessary to reduce a dose of Kanamycinum or to stop its use.
Patients with infectious and inflammatory diseases of urinary tract should accept the increased amount of liquid.
Aminoglycosides get into breast milk in a small amount (the complications connected using drug at babies were not registered as active agent is badly soaked up from digestive tract).
In the absence of positive clinical dynamics it is necessary to consider a possibility of development of resistance of microorganisms. In these cases it is necessary to cancel drug and to begin performing the corresponding therapy.
Medicinal interaction
Kanamycinum pharmaceutical is incompatible with gentamycin, streptomycin, penicillin, Monomycinum, heparin, kapreomitsiny, cephalosporins, erythromycin, Amphotericinum In and nitrofurantoin.
At simultaneous use of Kanamycinum with some medicines there can be undesirable effects:
- Polymyxins, Acidum nalidixicum, Vancomycinum, Cisplatinum: increase in probability of development nefro-and ototoxicity;
- Penicillin, cephalosporins, diuretics (especially furosemide), streptocides, non-steroidal anti-inflammatory drugs: blocking of elimination of aminoglycosides, increase in their concentration in blood serum that leads to strengthening nefro-and a neurotoxicity;
- Indometacin (parenteral administration): increase in risk of development of toxic action of Kanamycinum (increase in T1/2 (elimination half-life) and decrease in clearance);
- Anti-myasthenic medicines: decrease in their effect;
- Kurarepodobny drugs, general anesthetics and polymyxins: strengthening of their myorelaxation action;
- Polymyxins for parenteral administration, метоксифлуран and other medicines blocking neuromuscular transmission (opioid analgetics, halogenated hydrocarbons as drugs for inhalation anesthesia), transfusion of large amounts of blood with citrate preservatives: increase in risk of an apnoea (because of strengthening of neuromuscular blockade).
Terms and storage conditions
To store in protected from light, the place, unavailable to children, at a temperature up to 25 °C.
Period of validity – 2 years.
Work which to the person not to liking, is much more harmful to his mentality, than lack of work in general.

It would seem, about it there can be no disagreements: water is necessary for a human body for normal zhiznedeyatet...
Section: Articles about health
All parents are ready to what the baby often and pisat much. Since then, as the absorbing diapers strongly became current, keeping of the kid in dryness does not represent any problems. But if the grown-up kid continues to urinate in panties, parents of a nacha...
Section: Articles about health
Within several decades of our compatriots convinced that the use of butter nasty affects a condition of coronary vessels. As a result the reputation of a product was impaired thoroughly a little, and many almost ceased to include it in the diet, having given preference "to safer" to vegetable fats. Meanwhile, the last researches showed that harm of butter for health is strongly exaggerated. But the product has a number of unique properties, to...
Section: Articles about health
Not without reason doctors say that 90% of diseases begin or develop because of misoperation of intestines. Disturbance of its functions связ...
Section: Articles about health
Turnip, radish, horse-radish – once these and other products enjoyed wide popularity at our ancestors, being not only the food sating an organism but also the medicines curing of many diseases. Unfortunately, having given the use of some of them...
Section: Articles about health
All got used long ago that, having addressed the plastic surgeon, it is possible to modify natural parameters of a figure or to minimize the damages put to appearance with ruthless time. Many people (preferential women) worldwide annually decide on operations such. However there are also much more exotic interventions which are carried out seldom so far and cost expensive very much. We bring the story about the most unusual of them to your attention....
Section: Articles about health
The modern person not always manages to find housing in the environmentally friendly region and such work which would not do harm здо...
Section: Articles about health
Epilepsy is one of widespread neurologic diseases. Parents, whose children suffer from this illness, should face rumors and delusions, many of which remained since the Middle Ages....
Section: Articles about health
One of the major chemical processes happening in a human body are oxidation reactions. They go with participation of fats and carbohydrates which we receive from food, and the oxygen getting to us from air. A main goal of such reactions is obtaining the energy necessary for life activity. Unfortunately, as a result of these processes dangerous by-products – so-called free radicals are allocated. To minimize harm which they can cause to the person neo...
Section: Articles about health
The climax, or menopause is the normal process of the termination of genital function of the woman which is followed serious hormonal...
Section: Articles about health
It is known that the person for 80% consists of water which participates in all processes of an organism. The person loses liquid daily – as a result of sweating, breath, an urination, and its insufficient completion due to various reasons can bring to обезвожив...
Section: Articles about health
Urogenital candidiasis (milkwoman) – a fungal infection which annoys unpleasant feelings in the field of generative organs, being followed by white curdled allocations, an itch, discomfort during an urination, pain. She is called by Candida fungus – the opportunistic organism living on mucous membranes of an organism....
Section: Articles about health
It is pleasant to state a possibility of improvement of quality of life of people with problems of functioning of secretory system. By efforts that...
Section: Articles about health
A lot of things depend on a condition of a backbone in a human body, a backbone - not only a support for a body, it also a receptacle for a spinal cord, that is why malfunctions with a backbone are so dangerous. To treat rachis diseases very difficult and long...
Section: Articles about health
The unpleasant feelings connected with spring breakdown are familiar almost to each of us. Often happens that in March-April on the person weakness leans: he suffers from drowsiness, complains of bad mood, loss of interest in life and failures in affairs....
Section: Articles about health
The brain of the person is studied not one hundred years, but the quantity of the riddles connected with this body increases rather, than reducing...
Section: Articles about health
Eyes – unique body on the structure thanks to which the person obtains about 80% of information on the world around: about a form, color, size, the movement, and also many other parameters of objects or phenomena. But whether much we know about the most valuable body...
Section: Articles about health
The trophic ulcer is not an independent disease. This heavy complication arising owing to a thermal injury (a burn or a frostbite), chronic pathologies of arteries or veins of the lower extremities, a diabetes mellitus, and also some defeats of connecting fabric, absorbent vessels, skin or nervous trunks. Pathology is shown in the form of not healing wound located on the internal surface of a shin, a foot sole, a heel or toes....
Section: Articles about health
It is difficult to revaluate importance of kidneys for an organism. These bodies not only perform work on purification of blood of decomposition products and выв...
Section: Articles about health
Maternal milk is the best food for the newborn. It is the unique natural product containing an optimum set of nutrients, and which is best adapted in order that the baby normally developed and it was protected from harmful fa...
Section: Articles about health
Frosty air, fresh wind and easy snowball at most of Russians are associated with cheerfulness, health and cheerful entertainments on which our winter is so generous. But, unfortunately, cold season sometimes brings also troubles with health. It is not about seasonal colds and frostbites, and about those chronic illnesses which symptoms are shown preferential in the winter....
Section: Articles about health
Small appetite at the child – the complaint which pediatricians should hear practically from each mother. Most often it is carried to разр...
Section: Articles about health
Antibiotics - - it is possible to call the chemical compounds suppressing growth of bacteria the break in the field of medicine which allowed to save mankind from many diseases incurable earlier: tuberculosis, plague, syphilis and many others. A contribution of drugs to rescue of people from...
Section: Articles about health
What is in our understanding weeds? It plants which are considered to be suitable only for compost pits and feeding of animals. Meanwhile, among the weeds growing literally under legs it is possible to find the mass of the officinal herbs having invaluable advantage for human health. It is possible not only to be treated by most of them, using as broths, tinctures, compresses, but also to accept in food as usual products. Let's consider 8 widespread and often ignored by people...
Section: Articles about health
For the person who daily since morning gathers for work it is very important to wake up vigorous and ready by day of work. On most...
Section: Articles about health
Let's begin with the fact that a separate illness which is called "adjournment of salts", just does not exist. In practice this household name of disbolism leading to development of a number of diseases. Pathological process consists that in an organism проис...
Section: Articles about health
Water with a lemon - idle time in preparation drink which supporters of a healthy lifestyle already managed to appreciate. Used in a warm look and on an empty stomach, it is one of the most useful prophylactics allowing to prevent tens of diseases and just to raise an organism tone. Especially effectively to use warm water with lemon juice after a serious illness, during a season of the colds, and also to children, old men and pregnant women which do not have contraindications...
Section: Articles about health