





Pegintron
Application instruction:
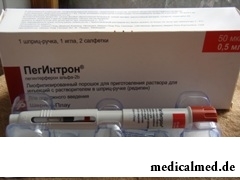 Pegintron – the antiviral, immunostimulating, immunomodulatory drug of peginterferon alpha 2b.
Pegintron – the antiviral, immunostimulating, immunomodulatory drug of peginterferon alpha 2b.
Form of release and structure
Dosage form of drug – lyophilisate for preparation of solution for hypodermic (п / to) introductions: powder of almost white or white color without foreign inclusions complete with solvent: water for injections – the transparent colourless liquid which is not containing visible particles (on 0,05; 0,08; 0,10 or 0,12 mg in glass bottles of 2 ml, complete with 0,7 ml of solvent in an ampoule of 2 ml, 1 set in packaging; on 0,05; 0,08; 0,10; 0,12 or 0,15 mg from 0,7 ml of solvent in two-chamber the syringe handles, complete with a needle for п / to injections and 2 napkins for processing of skin and a membrane the syringe handle in an injection site, 1 set in packaging).
The excess amount of solvent is necessary for compensation of losses in the course of dissolution of lyophilisate and at administration of ready solution.
Active ingredient – peginterferon alpha 2b:
- Bottle: in 0,5 ml of ready solution – 0,05; 0,08; 0,10 or 0,12 mg;
- Syringe handle: in 0,5 ml of ready solution – 0,05; 0,08; 0,10; 0,12 or 0,15 mg.
Auxiliary components of solution: sodium dihydrophosphate, sodium hydrophosphate, polysorbate 80, sucrose.
Indications to use
Pegintron apply to therapy of the chronic hepatitises B and C at patients 18 years, in the absence of liver diseases at them in a decompensation stage are more senior.
In medical practice it is considered to be optimum treatment of chronic hepatitis With a combination of a ribavirin with drugs of interferon alpha 2b, including peginterferon alpha 2b. At such combination therapy it is necessary to observe recommendations about use of a ribavirin also.
Contraindications
- Autoimmune a disease in the anamnesis, including autoimmune hepatitis;
- Serious mental illness or the expressed mental disorders in the anamnesis, including suicide thoughts or attempts and a heavy depression;
- Serious illness of cardiovascular system, unstable or uncontrollable throughout previous half a year;
- Dysfunctions of a thyroid gland at which it is not possible to normalize its work with the help of medicamentous therapy;
- Renal failures with the clearance of creatinine (CC) of ≤50 ml/min. (for use in combination with ribaviriny);
- Liver diseases in a decompensation stage;
- Epilepsy, dysfunctions of the central nervous system (CNS);
- Pregnancy, including that at the woman to whose partner male the combination therapy Pegintron with ribaviriny is necessary;
- Period of breastfeeding (lactation);
- Children's and teenage age up to 18 years (in view of lack of experience of use);
- Hypersensitivity to any interferon, including to peginterferon alpha 2b, and to other components of drug.
With care apply peginterferon alpha 2b at diseases with the increased risk of an invalidism: pulmonary diseases, including chronic obstructive pathologies; a diabetes mellitus with tendency to development of ketoacidosis; disturbance of coagulability of blood (thrombophlebitis, thrombembolia of a pulmonary artery); the expressed miyelosupressiya.
Route of administration and dosage
Treatment by Pegintron there has to begin the doctor having experience with patients with hepatitis B and C, further therapy also has to be carried out under its control.
The drug is administered subcutaneously, every time choosing the new place for an injection.
The dose is chosen individually and depends on safety of use and the predicted efficiency of peginterferon alpha 2b. In the course of treatment the dose is adjusted if undesirable reactions are observed or data of laboratory indicators change; in case of preservation of undesirable effects or their repeated emergence after carrying out dose adjustment, therapy is stopped.
For treatment of chronic hepatitis B the dose of drug is defined at the rate of 0,001-0,0015 mg/kg of body weight, injections do 1 time in 7 days on an extent of half a year about one year. At the chronic hepatitis B which is difficult giving in to treatment caused by a virus of a genotype of C or D to reach necessary therapeutic effect can be required to use higher doses and/or more long course.
For treatment of chronic hepatitis C (monotherapy) the dose of drug is defined at the rate of 0,0005 or 0,001 mg/kg of body weight, injections do 1 time in 7 days for, at least, half a year. If after the first half a year of treatment of RNA the virus from serum eliminirut, then the course needs to be continued for half a year that in general will make 1 year. When later half a year of RNA the virus is still defined in serum, therapy should be stopped.
At a combination therapy of chronic hepatitis alpha 2b with ribaviriny Pegintron's dose decides On peginterferon at the rate of 0,0015 mg/kg of body weight, injections do 1 time in 7 days.
Ribavirin (capsule of 200 mg) accept inside daily, along with meal, in the daily dose calculated depending on body weight:
- To 65 kg: 800 mg (4 capsules) – 2 pieces in the morning and 2 pieces in the evening;
- From 65 to 85 kg: 1000 mg (5 capsules) – 2 pieces in the morning and 3 pieces in the evening;
- More than 85 kg: 1200 mg (6 capsules) – 3 pieces in the morning and 3 pieces in the evening.
The mode of dosing of Pegintron and ribavirin at a combination therapy of chronic hepatitis C calculated depending on body weight; a dosage of a syringe handle/bottle (mg / 0,5 ml) / a dose for administration of peginterferon alpha 2b once a week (ml) / a daily dose of a ribavirin (mg)/quantity of capsules of 200 mg (piece):
- To 40 kg: 0,05/0,5/800/4 (in the 2nd morning + in the 2nd evening);
- From 40 to 50 kg: 0,08/0,4/800/4 (in the 2nd morning + in the 2nd evening);
- From 51 to 64 kg: 0,08/0,5/800/4 (in the 2nd morning + in the 2nd evening);
- From 65 to 75 kg: 0,1/0,5/1000/5 (in the 2nd morning + in the 3rd evening);
- From 76 to 85 kg: 0,12/0,5/1000/5 (in the 2nd morning + in the 3rd evening);
- More than 85 kg: 0,15 (only syringe handle)/0,5/1200/6 (in the 3rd morning + in the 3rd evening).
The recommended treatment duration depending on a virus genotype:
- Virus of a genotype 1: if after 3 months of treatment virus RNA elimination from blood serum is not noted, then at continuation of therapy emergence of the resistant virologic answer is very improbable. Patients with the virologic answer after 3 months of treatment should continue a course on an extent of 9 months (the general duration of therapy about 12 months). At low concentration of a virus (no more than 2 million copies/ml) if after monthly therapy there was a virus RNA elimination, and it did not come to light in the subsequent period, then after 6 months therapy can be stopped (course duration – 6 months) or is continued for 6 months (course duration – 12 months); but it is necessary to consider that the risk of a recurrence after a course lasting 6 months is higher, than after a 12-month course;
- Virus of a genotype 2 or 3: for all patients of this group therapy course duration – is recommended 6 months;
- Virus of a genotype 4: it is noted that patients of this group hardly will respond to treatment; according to clinical observations of group of 66 patients it is revealed that 4 it is possible to apply tactics of therapy of a virus of a genotype to treatment of a virus of a genotype 1.
In case of significant undesirable reactions or deviations of these laboratory indicators at monotherapy by peginterferon alpha 2b or during complex treatment by peginterferon alpha 2b and ribaviriny correction of dosing or cancellation of administration of drugs before the termination of undesirable effects is required.
At monotherapy the dose of peginterferon alpha 2b is reduced to a half therapeutic if indicators of number of neutrophils less 750/mkl, and thrombocytes less than 50 000/mkl; injections of drug stop when indicators of number of neutrophils less 500/mkl, and thrombocytes less than 25 000/mkl.
At a combination therapy of peginterferon alpha 2b with ribaviriny the doctor has to carry out correction of doses. If after change of doses portability of treatment does not improve, Pegintron's use and/or a ribavirin is required to be stopped.
Monotherapy at a renal failure, dose adjustment:
- The renal failure of moderate severity, at KK of 30-50 ml/min. – an initial dose of peginterferon alpha 2b decreases by 25%;
- The renal failure of heavy degree, at KK of 10-29 ml/min. (including patients on a hemodialysis) – an initial dose of peginterferon alpha 2b decreases by 50%.
At increase in content of creatinine in serum more than 2 mg/dl a course need to be interrupted.
Therapy Pegintrony and ribaviriny at patients with easy degree of a renal failure, at KK ≥ 50 ml/min. can cause development of anemia; at patients at KK ≤ 50 ml/min. the combination therapy should not be carried out.
The lyophilisate and solvent which are in the syringe handle mix up just before introduction by the technique described in the enclosed leaf insert.
Pegintron in bottles it is necessary to part only with the solvent enclosed in a set: sterile syringes of 0,7 ml of water for injections need to enter into a bottle with lyophilisate which should be stirred up carefully before full dissolution of powder; lyophilisate dissolution time – no more than 10 minutes (usually quicker); in the sterile syringe gather a required dose (to 0,5 ml) solution; it is impossible to mix peginterferon alpha 2b with other medical substances / drugs.
Before introduction ready solution needs to be examined: it has to be colourless, transparent, not contain visible particles. At discoloration or emergence of visible inclusions it is not necessary to use solution. The ready drug is recommended to be administered immediately, and in the absence of such opportunity to store at a temperature of 2-8 °C no more than 24 watch. The solution which remained after an injection is not subject to use further, it should be utilized according to the existing requirements.
Side effects
Side effects owing to monotherapy by peginterferon alpha 2b generally easy or moderately expressed, do not demand the termination of therapy:
- Most often (more than 10%): pain and an inflammation in an injection site, a headache, dizziness, increased fatigue, sleeplessness, irritability, fever, a fever, a depression, feeling of alarm, nausea, an alopecia, grippopodobny symptoms, a joint pain, musculoskeletal pains, an abdominal pain, diarrhea, an adynamy, pharyngitis, anorexia, decrease in body weight, disturbance of concentration of attention;
- Less often (from 2% to 10%): a xeroderma, an itch, a febricula, perspiration, rash, pain in right hypochondrium, apathy, emotional lability, confusion of consciousness, excitement, nervousness, viral infections, cough, short wind, drowsiness, a stethalgia, changes of a thyroid gland, dyspepsia, paresthesias, a hypertension, a hyperesthesia, a hypesthesia, a neutropenia, sight misting, abdominal distention, decrease a libido, an erythema, an unstable chair, a lock, dryness in a mouth, vomiting, eye pain, conjunctivitis, a nose congestion, sinusitis, a menorrhagia, inflows, menstrual disturbances;
- Seldom: serious problems from TsNS (including suicide thoughts and attempts), psychosis (including hallucinations), the agressive behavior sometimes aimed on people around; and also peripheral neuropathy, attacks, gipertriglitseridemiya, pancreatitis, arrhythmia, diabetes.
Besides, at 4% of the patients receiving Pegintron in a dose of 0,0005 mg/kg and 7% of the patients receiving 0,001 mg/kg the granulocytopenia (<750/mkl), and at 1% and 3% (respectively) – thrombocytopenia was observed (<70 000/mkl).
Side effects against the background of a combination therapy peginterferon alpha 2b with ribaviriny:
- Most often (from 5% to 10%): rhinitis, food faddism, tachycardia;
- Less often (from 2% to 5%): defeat of the lacrimal gland, thirst, a faint, arterial hypotension, arterial hypertension, an agressive behavior, heartbeat, a tremor, a glossitis, stomatitis, bleeding of gums, a stomacace, a sonitus, a disturbance/hearing loss, average otitis, eczema, a fungal infection, respiratory disturbances, bronchitis, a rhinorrhea, reactions of hypersensitivity by a sunlight, prostatitis, a limfoadenopatiya, the increased fragility of hair;
- Very seldom: aplastic anemia.
Both at monotherapy, and at the combined treatment alpha 2b with ribaviriny can be observed by peginterferon:
- Seldom: ophthalmologic pathologies, including retinopathies (including a papilledema), obstruction of veins or arteries of a retina, a retinal apoplexy, focal changes, restriction of fields or decrease in visual acuity, an optic neuritis; cardiovascular disturbances, including arrhythmias (presumably connected with previous diseases and with the therapy by means with cardiotoxic action which was carried out earlier); the cardiomyopathy at the patients who did not have data on diseases of cardiovascular system in the anamnesis can be reversible after completion of therapy interferon an alpha;
- Very seldom: рабдомиолиз, a renal failure, a renal failure, a miositis, a myocardial infarction, heart ischemia, brain ischemia, brain hemorrhage, ulcer or ischemic colitis, encephalopathy, a sarcoidosis (a sarcoidosis aggravation), Stephens-Johnson's syndrome, a mnogoformny exudative erythema, a toxic epidermal necrolysis, a necrosis (a necrosis of fabrics) in an injection site.
Owing to use of interferon an alpha various autoimmune pathologies, and also the disturbances mediated by immune system including the idiopathic Werlhof's disease (IWD) and the trombotichesky Werlhof's disease (TWD) were noted.
Special instructions
At heavy mental disturbances (including data in the anamnesis) treatment can be begun only after careful personal inspection and passing of the corresponding treatment of a mental disorder.
Heavy disturbances from TsNS are in certain cases observed, including at symptoms of a depression, during therapy by Pegintron, especially at the patients of advanced age accepting high doses of drug. Considering potential complexity of similar undesirable effects it is recommended to provide continuous monitoring of patients during therapy and for 6 months after its termination. Generally these phenomena are reversible, but some patients can be necessary for a complete recovery up to 3 weeks after the termination of reception of peginterferon alpha 2b. In case of preservation or increase of symptoms in the course of treatment, especially suicide intentions, the depression, an agressive behavior, is required to interrupt a course and to provide the timely address to the psychiatrist.
Patients with heart failure, arrhythmias, a myocardial infarction (including data) need to be in the anamnesis under constant medical observation; prior to the beginning of and during therapy it is recommended to make the electrocardiogram (ECG). At arrhythmias (generally supraventricular) their usual treatment, as a rule, suffices, but drug withdrawal in exceptional cases can be required.
At allergic reactions of immediate type (a small tortoiseshell, a Quincke's disease, a bronchospasm, an anaphylaxis) Pegintron it is necessary to cancel and to immediately appoint an adequate symptomatic treatment; cancellations of therapy do not demand passing rashes.
Prior to therapy it is recommended to investigate function of kidneys at all patients, for patients with a renal failure during therapy it is required to conduct careful observation, in case of need to adjust Pegintron's dose on reduction.
At the first signs of a decompensation of diseases of a liver it is necessary to interrupt therapy.
Fever can be the accompanying manifestation of a grippopodobny syndrome, frequent side effect against the background of treatment by interferon, but at a resistant feverish state it is required to exclude other reasons of its emergence.
Adequate hydration of the patient is necessary to avoid the hypotension connected with reduction in a liquid volume organism; perhaps replaceable administration of liquid will be necessary.
In rare instances against the background of therapy by Pegintron in lungs formation of infiltrates of not clear etiology, pneumonites or pneumonia, including with a lethal outcome was noted. Therefore in cases of emergence of cough, fever, an asthma, other respiratory symptoms patients should do a thorax X-ray analysis. If on the roentgenogram of lungs infiltrates or signs of pulmonary insufficiency are visible it is required to provide their monitoring, and in case of need – to cancel drug. Similar reactions are more characteristic of the patients receiving interferon an alpha with chronic hepatitis C, but also registered them at therapy of oncological patients. Immediate cancellation of Pegintron and therapy by glucocorticosteroids (GKS) lead to treatment of side effects from lungs.
Emergence of autoantibodies and clinical displays of autoimmune pathologies arise probably at therapy by interferon of the patients predisposed to autoimmune disorders more often.
At complaints of the patient to restriction of fields of vision or decrease in visual acuity it is required to conduct careful ophthalmologic examination. More often similar undesirable effects arise in case of associated diseases therefore at a diabetes mellitus or arterial hypertension prior to therapy by Pegintron it is necessary to undergo inspection at the ophthalmologist.
Pathological changes of peridental fabrics and teeth at patients were registered during a combination therapy peginterferon alpha 2b and ribaviriny. Their prolonged combined use causes dryness in a mouth that can promote destruction of teeth and injury of a mucous oral cavity. During therapy it is necessary to brush teeth twice a day and to regularly undergo sanitation. After vomiting it is necessary to rinse a mouth carefully.
At therapy of chronic hepatitis C cases (2,8%) of dysfunction of a thyroid gland – a hyperthyroidism or a hypothyroidism which were controlled by means of standard treatment were noted. The mechanism of influence of peginterferon alpha 2b on function of a thyroid gland is authentically unknown. It is recommended to determine prior to therapy at patients the level of thyritropic hormone in serum, and in case of any disturbances of work of a thyroid gland to use standard therapy. It is not necessary to use drug if such therapy does not maintain activity of thyritropic hormone at the normal level.
There are descriptions of episodes of an aggravation of a sarcoidosis and psoriasis at therapy by interferon alpha 2b therefore to use drug at patients with a sarcoidosis or psoriasis it is recommended only if the expected advantage of therapy is much higher than possible risk of complications.
Safety and efficiency of use of Pegintron are not up to the end studied, both at monotherapy, and in a combination with ribaviriny, at recipients at organ transplantation. By results of preliminary data increase of episodes of rejection of the replaced kidney is noted, there are also messages on rejection of a transplantirovanny liver, but reliable relationship of cause and effect between rejection of transplanted organs and reception of interferon an alpha is not established.
All patients prior to therapy and in the course of treatment are recommended to do the general and biochemical blood tests, the following values of indicators are admissible: neutrophils> 1500/mkl, thrombocytes> 100 000/mkl. Besides, it is necessary to watch the level of lipids in blood as at use of interferon alpha 2b cases of a gipertriglitseridemiya and growth of triglycerides in a blood plasma sometimes expressed were noted.
In case of emergence against the background of Pegintron's use of such side reactions as drowsiness, fatigue, confusion of consciousness it is not recommended to manage a difficult technique or motor transport.
Medicinal interaction
Repeated combined use of Pegintron and ribavirin did not reveal between them signs of pharmacokinetic interaction.
Terms and storage conditions
To store in the place unavailable to children, at a temperature of 2-8 °C.
Period of validity – 3 years.
Name of drug
Price
Drugstore
Scientists from the Oxford university conducted a number of researches during which they came to a conclusion that vegetarianism can be harmful to a human brain as leads to decrease in its weight. Therefore scientists recommend not to exclude completely from the diet fish and meat.

Subfebrile temperature call fervescence to 38 degrees, and subfebrile condition - existence of such temperature from above...
Section: Articles about health
All know that self-treatment is dangerous. However absolutely it is almost impossible to do without it. Rate of modern life does not allow to handle each small trouble to the doctor and information on ways of independent rendering medical the help...
Section: Articles about health
Summer in the heat. Many are going to spend vacation abroad. Travelers the tender seas, rest on beaches wait, for sightseeing, campaigns on natural and cultural reserves. But, unfortunately, on vacation also problems with health can wait for us. On a foreign trip it is possible to face also diseases which not only will spoil long-awaited issue, but also will force to be treated within long months after its termination. To be insured completely from troubles of it a sort...
Section: Articles about health
What will only not be thought up by persons interested to have a beautiful figure. Here the last innovation – for weight loss needs to be eaten greasy food. Give ра...
Section: Slideshow
Ability of an organism to resist to adverse environmental factors (to impact of temperature drops, humidity and pressure, to the attacks of causative organisms, etc.) directly depends on what the person eats. Business here not only in, that C...
Section: Articles about health
The kid who was recently born is surrounded with love of adult family members and their cares without which the baby cannot exist. Some parents consider that gentle attachment and caress are quite enough that the child correctly developed and was happy, but it not so. It is important to know as much as possible about specifics of care of the baby, the reasons of his behavior and possible problems. Only the "able to see" love will provide to the little man that it is necessary for him....
Section: Articles about health
It is impossible to imagine human life in which there would be no plants. Practically in each apartment and any of productions...
Section: Articles about health
Work of a brain is extremely complex and in many respects is not studied yet. It is confirmed also by the features of thought processes which are shown when the person sleeps. Let's tell about some of them....
Section: Articles about health
The phenomenon of improvement of a condition of the patients at administration of drugs who are not containing active agents, so-called effect of placebo is known long ago. At the end of the 18th century the American doctor Perkins began to treat people the "miracle" sticks made of alloy of steel and brass. Was for several minutes to press such subject enough to a sore point that it became much easier for the patient. Having suspected Perkins of charlatanism, his colleagues tried to repeat "miracle" by means of sticks, steles...
Section: Articles about health
You are office worker, the driver, the fan of winter sports or do not think of life without bicycle? You conduct a slow-moving image жизн...
Section: Articles about health
Each person supports all life a SARS about 200 times. The peak of incidence falls on cold season, but it is possible to get sick with a temperature and a pharyngalgia, and sometimes and very possibly, even during a heat. The reasons for development of catarrhal diseases exists множество:...
Section: Articles about health
Vitamin complexes belong to the most popular drugs, probably, in our country there is no person who was not hearing about advantage of vitamins and never their accepting. The more vitamins, the better, we consider and as it appeared, cruelly we are mistaken. Whether vitamins, whether so harmlessly general hobby for polyvitaminic complexes and whether it is possible to do without them are so useful? Let's try to understand....
Section: Articles about health
Kidneys perform the most important function of clarification of blood from those products of metabolic processes which cannot be used орг...
Section: Articles about health
The immunity role in growth of the child is invaluable. The proteins-immunoglobulins produced by immune system preserve the child against the diseases capable − owing to an organism weak still − to serve as a stressful factor, to become the reason of many complications and delays in unless...
Section: Articles about health
The medicine promptly develops, and the fact that else quite recently it seemed by miracle can now. We are not surprised any more to the fact that people with artificial joints and extremities can play sports, organ transplantation became a routine, and the latest cancer medicine allowed to achieve reduction of mortality in tens of times. Miracles of plastic surgery thanks to which people in 60 years are in the flower of beauty and freshness, too not a sensation any more....
Section: Articles about health
Healthy lifestyle today in fashion, and many parents think of that the child from the early childhood played sports. To a Torah...
Section: Articles about health
For many women the word "fat" sounds as a sentence. In aspiration to an ideal figure they try to exclude, first of all, from the menu all dishes containing fats without having at the same time a clear idea of a role of these substances in exchange processes, and about an afterbirth...
Section: Articles about health
Radiological methods of a research are applied in medicine more than hundred years, and thanks to them millions of lives were saved. In many cases without X-ray it is impossible to make exact idea of a condition of bodies and fabrics, it is correct to make the diagnosis. Nevertheless, many myths about researches such continue to exist. Let's consider the most widespread of them....
Section: Articles about health
Quite large number of people adheres to the principles of vegetarian food. But how to be if in a family of vegetarians is д...
Section: Articles about health
Tick-borne encephalitis – one of the most dangerous viral diseases which causative agents transfer and is given to people by ixodic mites. These are the small blood-sicking insects living in the considerable territory of our country. The person bitten by a tick can catch...
Section: Articles about health
Water with a lemon - idle time in preparation drink which supporters of a healthy lifestyle already managed to appreciate. Used in a warm look and on an empty stomach, it is one of the most useful prophylactics allowing to prevent tens of diseases and just to raise an organism tone. Especially effectively to use warm water with lemon juice after a serious illness, during a season of the colds, and also to children, old men and pregnant women which do not have contraindications...
Section: Articles about health
Stability of a hormonal background is one of the most important conditions of preservation of health of the woman. At the same time endocrine system –...
Section: Articles about health
Statistically, pathologies of a thyroid gland in the world more than 500 million people have. Failures in work of this body lead to heavy disbolism, development of heart diseases, vessels, a reproductive and nervous system. In hard cases excessive...
Section: Articles about health
Cellulitis - very widespread cosmetic shortcoming which arises approximately at 80% of women sooner or later. Emergence it is connected with change of structure of a hypodermic fatty layer. At the same time on the surface of skin at first there are roughnesses (cambers and cavities), and then small consolidations, the so-called effect of an orange-peel is shown. Changes in a condition of hypodermic cellulose are a consequence of a hormonal imbalance in an organism....
Section: Articles about health
History of mankind contains several tens of epidemics whose emergence was compared by eyewitnesses and historians to doomsday. With...
Section: Articles about health
The advantage of swimming for the person is so high that this sport is not only the most popular, but also is widely applied in medicine and rehabilitation processes. If you look for for yourself the occupation allowing pleasantly and to spend time, then swimming with advantage...
Section: Slideshow
It is known that the person for 80% consists of water which participates in all processes of an organism. The person loses liquid daily – as a result of sweating, breath, an urination, and its insufficient completion due to various reasons can lead to dehydration of varying severity. Dehydration (dehydration) occurs already in case of loss of liquid in number of 1% of body weight and can result both in easy thirst, and by the death. In time to notice signs обезвож...
Section: Articles about health

