





Alvesko
Application instruction:
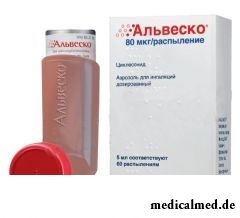 Alvesko – an inhalation glucocorticosteroid (GKS) for treatment of bronchial asthma.
Alvesko – an inhalation glucocorticosteroid (GKS) for treatment of bronchial asthma.
Form of release and structure
Dosage form – an aerosol for inhalations dosed: transparent colourless solution (in aluminum barrels with the dosing valve and the mouthpiece closed by a protective cover on 5 and 8 ml (60 or 120 sprayings respectively), 1 barrel in a pack cardboard).
Active agent: циклесонид, its content is to 1 dose (1 spraying) – 40, 80 or 160 mkg.
Auxiliary components: ethanol and норфлуран (HFA-134a).
Indications to use
Alvesko – means for therapy of bronchial asthma.
Contraindications
- Children's age up to 6 years;
- Individual hypersensitivity to drug.
With care:
- Active or chronic form of pulmonary tuberculosis;
- Virus, fungal or bacterial respiratory infections.
Route of administration and dosage
Alvesko is intended for peroral inhalation. It is necessary to use drug daily, prolonged treatment.
The initial dose is selected by the doctor depending on weight of a course of a disease. After achievement of necessary clinical effect the dose is reduced to the minimum, allowing to control manifestations disease.
To teenagers 12 years and to adult, including elderly people are more senior, at bronchial asthma easy and moderate severity appoint 160-640 mkg a day, divide the highest daily dose into 2 uses. At a severe disease increase in a dose up to 640 mkg 2 times a day daily is possible.
Improvement of a state is noted within 24 hours after Alvesko's inhalation. The maximum effect has to be reached in 2-3 months of therapy.
To children 6 years are more senior appoint 80-160 mkg of 1 times a day or on 80 mkg 2 times a day.
Important condition of effective treatment is continuousness of use of drug, even in case of lack of symptoms of bronchial asthma.
Alvesko can be applied as independently, and with a spacer. In the latter case it is recommended to choose AeroChamber Plus spacer.
At the heavy course of bronchial asthma at the adults and teenagers who are constantly receiving peroral GKS (for example, Prednisolonum), Alvesko's dose makes 640 mkg 2 times a day. Treatment duration – 10 days then the daily dose gradually (no more than on 2,5 mg every week) is reduced to achievement of the minimum effective.
In a stage of remission of a disease transfer of patients from peroral GKS on Alvesko is possible.
Instruction for use of an inhaler:
- To remove a protective cap from a spray, to check a mouthpiece outside and inside and to be convinced that it dry and pure;
- To turn an inhaler upside down, to arrange an index finger at the bottom of a barrel, a thumb – under a mouthpiece;
- If the inhaler new or was not used more than 1 week, to execute the first 3 pressing of the valve in air;
- To make aside (not in an inhaler) the maximum exhalation as far as it is possible;
- To place a mouthpiece in a mouth and to close around it lips;
- To begin a slow and deep breath and to press an index finger an inhaler top. To take care that drug could not pass through space between a mouthpiece and lips;
- To hold the breath, to take out a mouthpiece from a mouth, to remove a finger from an inhaler top. The breath should be held at least for 10 seconds, whenever possible – longer;
- To exhale slowly through a mouth (not through a mouthpiece);
- In need of acceptance of one more dose it is necessary to wait 30 seconds then to repeat points 4-8;
- To put on a protective cap a barrel, to densely close and record it on site.
The barrel does not need to be stirred up since the aerosol dissolved.
For hygiene:
- It is regularly necessary to clear a mouthpiece a dry napkin outside and inside;
- To wipe a surface with a small opening from where there is medicine, using the dry put napkin;
- Not to use water or other liquids for cleaning.
Side effects
- From the alimentary system: infrequently (> 1/1000, <1/100) – unpleasant smack in a mouth, nausea, vomiting; seldom (> 1/10 000, <1/1000) – dyspepsia, an abdominal pain;
- From cardiovascular system: seldom – increase in arterial pressure, and also the strengthened heartbeat (at simultaneous use of drugs which can have side effect on a cordial rhythm, for example, of theophylline or salbutamol);
- From respiratory system: infrequently – cough after inhalation, a dysphonia, a paradoxical bronchospasm;
- Infectious and parasitic diseases: infrequently – fungal infections of an oral cavity;
- From a nervous system: infrequently – a headache;
- From integuments: infrequently – skin rash, eczema;
- Allergic reactions: seldom – a Quincke's disease, hypersensitivity reactions;
- Local reactions: infrequently – dryness of a mucous membrane of an oral cavity and throat, feeling of irritation and irritation in a throat;
- System side effects: at use of drug in high doses for a long time – Cushing's syndrome and kushingopodobny symptoms, such as reduction of density of a bone, glaucoma, a cataract, delay of growth at children and teenagers, suppression of adrenal glands.
Directly after inhalation the paradoxical bronchospasm is possible. It is acute nonspecific reaction on any inhalation drugs and can be connected with active agent, auxiliary components or cooling owing to evaporation of propellant at use of the dosed inhalers. In most cases the bronchospasm is adverse reaction which takes place independently and does not demand the treatment termination.
Special instructions
On doctor's orders Alvesko can be applied at pregnancy and a lactation if the expected effect surpasses potential risks. Newborns, whose mothers during pregnancy received GKS, have to be under careful medical observation to exclude a hypoadrenalism.
Alvesko is not intended for treatment of the asthmatic status and other bad attacks of bronchial asthma which stopping requires carrying out intensive therapeutic measures.
The dose of drug should be reduced to patients who need peroral GKS.
The paradoxical bronchospasm and other symptoms of narrowing of bronchial tubes which developed right after inhalations stop high-speed bronchodilatory means. After that the doctor has to examine the patient and resolve an issue of expediency of continuation of therapy of Alvesko. It is possible only if the expected effect considerably surpasses risks. At the same time it is necessary to consider interrelation between severity of bronchial asthma and the general predisposition of the patient to acute bronchial reactions.
In case of development of an infection it is necessary to appoint antibiotics.
At the patients transferred from peroral GKS on Alvesko depression of function of bark of adrenal glands is possible for a long time, and also there is a risk of development of side effects of peroral GKS after their cancellation for some time. In this case it is recommended to control reserve function of bark of adrenal glands. At the same time it is necessary to consider probability of residual deterioration in function of bark of adrenal glands in critical situation (surgical or therapeutic) and in other individual cases caused by a stressful situation. Thereof it is necessary to begin the corresponding treatment of GKS.
At serious aggravations and in case of insufficiency of function of bark of adrenal glands it is necessary to increase Alvesko's dose, if necessary – to appoint peroral GKS.
At the patients receiving system GKS (Prednisolonum or its equivalent) in a high dose or for a long time suppression of function of adrenal glands therefore it is regularly necessary to control it is possible and to gradually reduce a dose of system GKS. Approximately after 1 week it is possible to exclude gradually this drug, daily lowering its dose by 1 mg. At reception of a daily maintenance dose more than 10 mg it is necessary to observe extra care and to consider that reasonable can be more considerable dose decline during week intervals.
Cases when after drug withdrawal patients badly felt, despite preservation or even improvement of respiratory function are known. Such patients need to be inspected on existence of adrenocortical insufficiency.
At transfer of patients from system GKS on Alvesko there is a probability of development of allergic reactions (for example, allergic rhinitis, eczema) which were suppressed by system drug earlier. In these cases performing symptomatic therapy by antihistamines, including for topical and external use, the containing GKS is shown.
As action of inhalation GKS on children at prolonged use is up to the end not established, during treatment it is necessary to watch development of growth of children constantly. If it is slowed down, it is necessary to reconsider therapy and to lower a drug dose to the minimum effective, allowing to control symptoms bronchial asthma.
Information on Alvesko's influence on the speed of reactions and ability to concentration there is no attention.
Medicinal interaction
It is not recommended to apply along with Alvesko CYP3A4 inhibitors, including potential.
Terms and storage conditions
To store in the place, unavailable to children, at a temperature up to 25 °C. Not to open a cylinder and not to subject it to heating higher than 50 ºС.
Period of validity – 3 years.
During life the average person develops neither more nor less two big pools of saliva.

Not without reason doctors say that 90% of diseases begin or develop because of misoperation of intestines. Disturbance of its functions связ...
Section: Articles about health
The state of health of the person in many respects depends on food. The organism will well function if during food it receive only useful substances, necessary vitamins and microelements. In this case there will be no problems with digestion, with лишн...
Section: Articles about health
Ayurveda - the most ancient tselitelsky practice which came to us from India. It represents the doctrine about maintenance of physical, psychological and moral health of the person by means of the complex of procedures including a diet, cleaning of an organism, breathing exercises, massage, and in case of a disease - and medicinal therapy. The healers practicing Ayurveda assign very important part to spices, and at the heart of Ayurvedic drugs, as a rule, there are they. It is considered that spices not of t...
Section: Articles about health
Partial and the more so full loss of hearing significantly reduces quality of life. Difficulties with communication lead to loneliness and замкн...
Section: Articles about health
The pancreas performs two functions in a human body: release of enzymes without which digestion of carbohydrates, fats and proteins, and a producing hormones is impossible. The most important of them - insulin, is the main participant of carbohydrate metabolism, a normal...
Section: Articles about health
The climax, or menopause is the normal process of the termination of genital function of the woman which is followed by serious hormonal changes in an organism. Usually the menopause begins at the age of 50-55 years, but characteristics of this process are very individual. Factors of earlier approach of a climax are irregular sex life, numerous abortions, addictions, existence of endocrine, autoimmune and gynecologic diseases, frequent stresses and excessive hobby of diets...
Section: Articles about health
To look healthy and means well-groomed not only to be pleasant to people around, but also to feel strong, sure and taken place. To Spa...
Section: Articles about health
Long time antibiotics were considered as a panacea from all diseases and were appointed even at insignificant symptoms of an infection. Even now not everyone knows in what force of antibiotics how and when they should be accepted. Let's discredit 7 popular myths about such drugs...
Section: Articles about health
Women quite often suffer from complexes concerning the sizes of the bust. Strangely enough, not too modest, and excessively curvy shapes become the reason of sincere discomfort sometimes. Except psychological problems, the big bust sometimes creates also quite notable malfunctions with health: his owner can feel muscular dorsodynias, feeling of constant fatigue and difficulty of breath. Over time excess loading leads to development of diseases позвоночн...
Section: Articles about health
For anybody not a secret that the modern person eats not as his ancestors. For the last 100 years in broad access appeared with...
Section: Articles about health
The healthy nutrition is the invariable principle of health and good health for long years of the woman. Nevertheless, in a diet at each stage of life there are the features allowing to support an organism by those substances which are most necessary...
Section: Articles about health
80% of women at least once to lives complained of discomfortable feelings to breasts, consolidations and nagrubaniye. These are mastopathy symptoms. The mastopathy is characterized by change of a ratio between ferruterous and connective tissue tissues of mammary glands. It can lead to formation of cysts (a cystous mastopathy), gland consolidation (a fibrous mastopathy), or a combination of these processes (a fibrous and cystous mastopathy)....
Section: Articles about health
More than a half of the married couples which faced prostatitis – leave. The new broadcast "Female View of Prostatitis" will help to learn...
Section: Articles about health
The kid who was recently born is surrounded with love of adult family members and their cares without which the baby cannot exist. Some parents consider that gentle attachment and caress are quite enough that the child correctly developed and was happy...
Section: Articles about health
Antibiotics - - it is possible to call the chemical compounds suppressing growth of bacteria the break in the field of medicine which allowed to save mankind from many diseases incurable earlier: tuberculosis, plague, syphilis and many others. The contribution of drugs to rescue of people from epidemics of dangerous infections is huge, however at careless use antibiotics are capable to cause to an organism serious damage. Negative action can be shown in the form of easing of immunity, disturbance of balance of microflora in кишеч...
Section: Articles about health
The drugs stopping or oppressing life activity of pathogenic microorganisms are widely applied in clinical practice with 4...
Section: Articles about health
The name of this disease precisely reflects the problem reason: it consists in the bra fastener pressure upon a certain zone of a back. At the same time one of vertebrae of chest department of a backbone is as if blocked and loses mobility, and falling on it is nude...
Section: Articles about health
The number of long-livers is very small. One person from 5 thousand lives up to age of 90 years, and the centenary boundary steps over only one of 20 thousand. However, doctors claim that each of us is quite able to affect own destiny. At the same time it is not so much about living as long as possible, how many about an opportunity to keep physical and intellectual activity and to avoid decrepitude. We will also talk about the ways helping to achieve this result today....
Section: Articles about health
Venereal diseases in medicine are called the infections which are transmitted preferential sexually, now they are so...
Section: Articles about health
What is in our understanding weeds? It plants which are considered to be suitable only for compost pits and feeding of animals. Meanwhile, among the weeds growing literally under legs it is possible to find the mass of the officinal herbs possessing invaluable Paul...
Section: Articles about health
Some people consider what for medicine of the 21st century of secrets in the field of health of the person almost does not exist. It absolutely not so. The more answers scientists receive, the more the most difficult questions are raised for them by life. Besides, there are diseases which are not explained with science in any way of which existence people know for 100-150 years. These diseases meet not so often, but from some of them nobody is insured....
Section: Articles about health
The advantage of swimming for the person is so high that this sport is not only the most popular, but also is widely applied in copper...
Section: Slideshow
Very often as a source of the infection which caused a disease serves our house - the place which a priori has to be safe. However disease-producing bacteria can perfectly feel not only in insanitary conditions, but also in our apartment if not осущ...
Section: Articles about health
Cold – a state known to everyone which is followed by cold, cough, high temperature, a pharyngalgia. Often the first that we begin to do in hope again to become healthy – to accept medicines which are not always harmless whereas it is easy to facilitate displays of a disease by means of natural means. They not only softly eliminate disease symptoms, but also enrich the weakened organism with useful substances. We present you 8 drinks which are successfully used for...
Section: Articles about health
High temperature - a frequent symptom of such widespread diseases as a SARS, quinsy, pneumonia, etc. To reduce heat, облег...
Section: Articles about health
The majority of gynecologic diseases prove three main signs, each of which speaks about need of a visit to the gynecologist. Certainly, it is possible to establish the exact diagnosis only after inspection, but on the basis of some signs it is possible пр...
Section: Articles about health
Obesity is called a disease of 21 centuries, for the last 100 years the number of the people suffering from excess body weight considerably increased. Statistically, on Earth already about 1,5 billion corpulent people, and 500 million from them have the extreme degree of completeness negatively affecting quality and duration of their life. What served as the reason of growth of stout persons on the planet? How not to get to their ranks? Let's consider five main premises for increase in body weight in conditions современнос...
Section: Articles about health
