





Klayra
Application instruction:
 Klayra – the combined hormonal contraceptive applied to the prevention of undesirable pregnancy.
Klayra – the combined hormonal contraceptive applied to the prevention of undesirable pregnancy.
Form of release and structure
Klayra is let out in the form of five types of tablets, film coated: biconvex, round, on cross section – from almost white till white color a kernel and dark yellow, pink, pale yellow, red or white color a cover (on 28 pieces in blisters from an aluminum foil / polyvinylchloride, on 1 or 3 blisters in the book folding bed with a reception calendar).
Tablets, film coated – 2 pieces: dark yellow, with a text of "DD" on one of the parties in the correct hexagon.
Active agent is a part of 1 tablet: oestradiol valerate, micro 20 – 3 mg.
Tablets, film coated – 5 pieces: pink, with a text of "DJ" on one of the parties in the correct hexagon.
Active agents are a part of 1 tablet:
- Oestradiol valerate, micro 20 – 2 mg;
- Диеногест, micro – 2 mg.
Tablets, film coated – 17 pieces: pale yellow, with a text of "DH" on one of the parties in the correct hexagon.
Active agents are a part of 1 tablet:
- Oestradiol valerate, micro 20 – 2 mg;
- Диеногест, micro – 3 mg.
Tablets, film coated – 2 pieces: red, with a text of "DN" on one of the parties in the correct hexagon.
Active agent is a part of 1 tablet: oestradiol valerate, micro 20 – 1 mg.
Tablets (placebo), film coated – 2 pieces: white, on one of the parties in the correct hexagon a text of "DT".
Auxiliary components (темно-желтые/розовые/бледно-желтые/красные/белые tablets respectively): monohydrate of lactose – 48,36/47,36/46,36/50,36/52,1455 mg; mg povidone 25 - 4/4/4/4/3,0545; prezhelatinizirovanny corn starch – 9,6/9,6/9,6/9,6/0 mg; corn starch – 14,4/14,4/14,4/14,4/24 mg; magnesium stearate – 0,64/0,64/0,64/0,64/0,8 mg.
Cover (темно-желтые/розовые/бледно-желтые/красные/белые tablets respectively): a gipromelloza – 1,5168/1,5168/1,5168/1,5168/1,0112 mg; a macrogoal 6000 – 0,3036/0,3036/0,3036/0,3036/0 mg; talc – 0,3036/0,3036/0,3036/0,3036/0,2024 mg; titanium dioxide – 0,584/0,83694/0,83694/0,5109/0,7864 mg; dye yellow iron oxide – 0,292/0,03906 mg (dark yellow/pale yellow tablets respectively); dye red iron oxide – 0,03906/0,3651 mg (pink/red tablets respectively).
Indications to use
Klayra is appointed for peroral contraception.
Contraindications
- Thromboembolisms and fibrinferments (arterial and venous) now or in the presence in the anamnesis (including a myocardial infarction, a thrombembolia of a pulmonary artery, a deep vein thrombosis, a stroke now or in the anamnesis);
- Existence of risk factors (multiple or expressed) arterial or venous thrombosis (including extensive surgical interventions with a long immobilization, the complicated diseases of the valve device of heart, uncontrollable arterial hypertension);
- The states preceding development of thrombosis (including stenocardia, the tranzitorny ischemic attacks) now or in the presence in the anamnesis;
- The diabetes mellitus proceeding with vascular complications;
- Bleeding from a vagina of the obscure genesis;
- Migraine with focal neurologic signs, including in the presence in the anamnesis;
- The pancreatitis which is followed by the expressed gipertriglitseridemiya (now or in the presence in the anamnesis);
- Hormonedependent malignant tumors, including the tumors of mammary glands or generative organs (confirmed or in the presence of suspicion of them);
- Malignant and benign tumors of a liver (now or in the presence in the anamnesis);
- Serious illnesses of a liver and liver failure (Klayra's use can be begun after normalization of indicators of function of a liver);
- Pregnancy or at suspicion on it;
- Hypersensitivity to drug components.
Drug should not be used in the presence of any of the specified diseases/states, in cases of their development during therapy Klayr should cancel.
In the presence of risks/states, any of diseases/factors, before Klayra's reception it is necessary to correlate to care potential risk with the expected advantage of its use (each case in an individual order):
- Hereditary Quincke's disease;
- Arterial hypertension, smoking, extensive injuries and surgical interventions, dislipoproteinemiya, obesity, disturbance of a cordial rhythm, migraine, diseases of valves of heart, long immobilization and other risk factors of developing of a thrombembolia and thrombosis;
- System lupus erythematosus, diabetes mellitus, gemolitiko-uraemic syndrome, disease Krone and ulcer colitis, drepanocytic anemia and other diseases at which disturbances of peripheric circulation can be observed;
- Diseases which arose for the first time or were aggravated during pregnancy or at the previous use of sex hormones, including cholestatic jaundice, herpes of pregnant women, a cholestatic itch, a chorea of Sidenkhem, a cholelithiasis, a porphyria, an otosclerosis with deterioration in hearing;
- Gipertriglitseridemiya;
- Puerperal period.
Route of administration and dosage
Klayra is accepted inside, washing down if necessary with water or other liquid, regardless of meal. Drug needs to be accepted every day (continuously) on 1 tablet a day approximately in at one time in the order specified in a calendar for 28 days then begin reception of tablets from new packaging.
As a rule, menstrualnopodobny bleedings begin during reception of the last tablets of calendar packaging. At some women they begin after the beginning of reception of tablets from new packaging.
If the woman did not use hormonal contraception earlier, Klayra begins to be accepted in the first day of a natural menstrual cycle.
Upon transition from other combined hormonal contraceptive, tablets begin to accept Klayra next day after reception of the last active (with the content of active agent). When using a transdermalny plaster or vaginal ring Klayra's reception needs to be begun in day of their removal.
Transition from use mini-was drunk it is possible to carry out in any day, from an injection method – in day of purpose of the next injection, from intrauterine system with release of progestogen or an implant – in day of their removal. During the first 9 days of reception of Klayra it is recommended to use in addition barrier method of contraception.
After the abortion which is carried out in the first trimester of pregnancy it is possible to apply Klayra at once without use of additional measures of contraception.
After the abortion which is carried out in the second trimester of pregnancy and after the delivery drug begins to be accepted for 21-28 day. If Klayra's reception is begun later, during the first 9 days it is necessary to use in addition barrier method of contraception. In cases if already the sexual contact took place, before therapy it is necessary to exclude pregnancy or to wait for approach of the first periods.
The passed inactive (white) tablets can be neglected. At the admission during 12 reception hours of active tablets contraceptive protection does not go down, and the passed medicine needs to be taken at once as soon as the woman remembers it. Further Klayra's reception is continued according to the usual scheme.
At a delay of reception of an active tablet longer than 12 hours, contraceptive protection can decrease. The passed pill needs to be taken at once as soon as the woman remembers it even if it means that it is necessary to take at the same time 2 pill. Further Klayra's reception is continued according to the usual scheme.
Depending on day in which the woman missed reception of 1 tablet longer than for 12 hours, it is necessary to follow the following rules:
- 1-2 day (tablet of dark yellow color): the passed pill should be taken immediately, and following – according to the usual scheme (even at reception of 2 tablets in one day);
- 3-7 day (tablet of pink color): within 9 next days it is necessary to use additional measures of contraception, method of tablets is continued according to the usual scheme;
- 8-17 day (tablet of pale yellow color): within 9 next days it is necessary to apply additional measures of contraception;
- 18-24 day (tablet of pale yellow color): it is necessary to begin administration of drug from new calendar packaging at once (with the first tablet), within 9 next days it is necessary to apply additional measures of contraception;
- 25-26 day (tablet of red color): the passed pill should be taken immediately, and following – in usual time (even at reception of 2 tablets in one day);
- 27-28 day (tablets of white color – placebo): it is necessary to continue Klayra's reception according to the usual scheme, having thrown out the passed tablet.
In one day it is allowed to take no more than 2 pill.
What more tablets (during the period from 3 to 24 day, especially with the maintenance of a combination of two active components) were passed by, and than day of the admission of administration of drug is closer to a phase of reception of inactive tablets, subjects a pregnancy high probability (in cases if within 7 days before the admission of a tablet there was a sexual contact).
In the absence of menstrualnopodobny bleeding at the end of the current calendar packaging / at the beginning of new calendar packaging, it is necessary to consider probability of pregnancy.
Absorption of active agents of drug at heavy gastrointestinal disturbances can be incomplete therefore it is recommended to apply additional contraceptive measures.
In cases if in 3-4 hours after reception of a tablet with the content of active agent vomiting develops, the recommendations relating to the passed tablets work. If the woman does not want to change the usual scheme of reception of Klayra, it is necessary to take the corresponding additional pill (tablets) from new packaging.
Klayra should not be accepted to women after approach of a menopause.
Side effects
At Klayra's use the following side effects can develop:
- Nervous system: often – a headache, including tension headache; infrequently – dizziness, decrease a mood/depression, decrease in a libido, mental disturbances, changes of mood; seldom – aggression, affective lability, nervousness, uneasiness, increase in a libido, disturbance of attention, a dysphoria, sleep disorders, a stress, concern, вертиго, paresthesias;
- Alimentary system: often – pains in a stomach (including swelling); infrequently – nausea, diarrhea, vomiting; seldom – a gastroesophageal reflux;
- Cardiovascular system: infrequently – increase in arterial pressure, migraine (with aura and without it); seldom – inflows of heat to the person, bleeding from varicose expanded veins, pains on the course of veins, lowering of arterial pressure;
- Reproductive system: often – discomfort and mammary gland pains, an amenorrhea, disturbances in nipples, a dysmenorrhea, nipple pains, a metrorrhagia; infrequently – increase and diffusion consolidation of mammary glands, a fibrous and cystous mastopathy, a dysplasia of an epithelium of a neck of uterus, a dispareuniya, dysfunctional uterine bleeding, a menorrhagia, pains in pelvic area, cysts in ovaries, a premenstrual syndrome, a leiomyoma of a uterus, allocation from a vagina, uterus spasms, dryness in vulvovaginal area; seldom – high-quality new growths in a mammary gland, a galactorrhoea, a lactocele, bleeding during the sexual intercourse, bleeding from a vagina, a delay of menstrualnopodobny bleeding, a hypomenorrhea, a rupture of an oothecoma, burning sensation in a vagina, vulval/uterine bleeding (including the vulvovaginal discomfort, a smell from a vagina smearing allocations);
- Liver: seldom – a focal nodular hyperplasia of a liver, increase in activity of alaninaminotranspherase;
- Musculoskeletal system: seldom – feeling of weight, muscular spasms, dorsodynias;
- Hypodermic cellulose and skin: often – an acne; infrequently – an alopecia, rash (including a menocelis), an itch (including pruritic rash, a generalized itch); seldom – neurodermatitis, allergic skin reactions (including urticaria, allergic dermatitis), dermatitis, a hloazma, a hirsutism, a hypertrichosis, seborrhea, pigmentation disturbance, not specified damages of skin, including feeling of stiffness of skin;
- Organ of sight: seldom – intolerance of contact lenses;
- Invasions and infections: infrequently – fungal infections, vagina candidiasis, not specified vagina infections; seldom – vulvovaginal fungal infections, multi-colored deprive, herpes, candidiasis, a syndrome of estimated histoplasmosis of eyes, a bacterial vaginosis, infections of urinary tract;
- Alimentary disturbances and metabolism: infrequently – increase in appetite; seldom – a gipertriglitseridemiya, a liquid delay;
- General symptoms: often – increase in body weight; infrequently – a body degrowth, hypostasis, irritability; seldom – an indisposition, pain behind a breast, a lymphadenopathy, fatigue.
Special instructions
Before Klayra's use it is necessary to estimate carefully contraindications to her appointment on the basis of the family anamnesis of the woman, the anamnesis of life, and also gynecologic and all-medical examination.
The maximum risk of developing of a venous thrombembolia is noted in the first year of reception of Klayra (preferential – for the first 3 months).
Thrombosis of other blood vessels (for example, mesenteric, hepatic, renal, vessels of a retina, brain veins and arteries) develops extremely seldom.
The risk of developing of a thrombembolia and thrombosis (arterial and/or venous) increases at smokers, with age, and also in the presence of the following diseases / states:
- Existence in the family anamnesis of instructions on a venous or arterial thromboembolism;
- Obesity (index of body weight more than 30 kg/m ²);
- Fibrillation of auricles;
- Dislipoproteinemiya;
- Arterial hypertension;
- Diseases of valves of heart;
- Migraine;
- Long immobilization;
- Extensive surgical interventions, any operations performed on the lower extremities, extensive injuries.
It is necessary to consider the increased risk of development of a thromboembolism during the puerperal period. Development of disturbances of peripheric circulation in women with a diabetes mellitus, a system lupus erythematosus, a gemolitiko-uraemic syndrome, chronic inflammatory diseases of intestines (a disease Krone or ulcer colitis) and a sickemia is also possible.
Increase in weight and frequency of migraine can be the basis for the immediate termination of reception of Klayra.
In rare instances at use of drug developed high-quality, and in extremely exceptional cases – malignant tumors of a liver. When developing severe pains in upper parts of a stomach, increase in the sizes of a liver or symptoms of intra belly bleeding when carrying out differential diagnosis it is necessary to exclude liver tumors.
The risk of development of pancreatitis is higher at women with a gipertriglitseridemiya.
At development of permanent, clinically significant increase in arterial pressure Klayra's reception needs to be cancelled.
In the presence of hereditary forms of a Quincke's disease drug can worsen or induce symptoms of a Quincke's disease.
Klayra's reception can influence results of some laboratory researches, including biochemical parameters of a thyroid gland, function of a liver, kidneys and adrenal glands, concentration of transport proteins in plasma, parameters of carbohydrate metabolism, a fibrinolysis and coagulation.
During Klayra's use, in particular in the first months of therapy, development of irregular menstrualnopodobny bleedings in the form of the smearing allocations or breakthrough uterine bleedings is possible. It is necessary to carry out assessment of any irregular menstrualnopodobny bleedings only later 3 menstrualnopodobny cycles (after the adaptation period).
At repetition of irregular menstrualnopodobny bleedings or if they arise for the first time after the previous regular cycles, it is necessary to conduct careful examination to exclude malignant new growths or pregnancy.
Klayra's efficiency can decrease at gastrointestinal disturbances, the admission of tablets with the content of active components, and also because of the accompanying medicinal therapy.
Medicinal interaction
Klayra's interaction with some other medicines can result in lack of contraceptive effect and/or development of breakthrough uterine bleedings. In particular, it is necessary to consider effect of simultaneous use of Klayra with such drugs as:
- Rifampicin, carbamazepine, Phenytoinum, Primidonum, barbiturates and, perhaps, also окскарбазепин, griseofulvin, топирамат, ритонавир, фелбамат, and also drugs with the maintenance of the St. John's Wort which is made a hole: increase in clearance of sex hormones;
- Nenukleozidny inhibitors of the return transcriptase (for example, not Virapinum) and their combinations, HIV protease inhibitors (for example, ритонавир): influence on hepatic metabolism;
- The drugs inducing microsomal enzymes, antibiotics: decrease in enterogepatichesky circulation of estrogen and, respectively, reduction of concentration of oestradiol (it is recommended for use of these drugs and within 28 days after their cancellation to apply other method of contraception or to use barrier methods of contraception);
- Grapefruit juice, azolny antifungal drugs, verapamil, Cimetidinum, diltiazem, antidepressants, macroleads: increase in concentration of a diyenogest in a blood plasma.
Terms and storage conditions
To store in the place, unavailable to children, at a temperature up to 30 °C.
Period of validity – 4 years.
Name of drug
Price
Drugstore
The 74-year-old resident of Australia James Harrison became blood donor about 1000 times. It has a rare blood group which antibodies help to survive the newborn with a severe form of anemia. Thus, the Australian saved about two million children.

All like to sing. Small children with pleasure are engaged in a vocal, not especially thinking of hit in a melody. Adults most often...
Section: Articles about health
The dietology, as well as other sciences, does not stand still. Food stuffs are exposed to comprehensive study, and scientists obtain new information on their properties and influence on a human body. Unfortunately, this reasonable and natural process from time to time д...
Section: Articles about health
The concept "gluten" (differently, a gluten) combines group of the proteins which are a part of rye, barley and wheat. For most of people the use of the food stuffs containing a gluten not only is safe, but also it is very useful. Nevertheless, there is a number of myths about negative effect which allegedly gluten has on health of the person....
Section: Articles about health
All diseases from nerves – in this joke a big element of truth, are said by doctors. Constant stresses lead body to decrease in protective forces...
Section: Articles about health
Very often as a source of the infection which caused a disease serves our house - the place which a priori has to be safe. However disease-producing bacteria can perfectly feel not only in insanitary conditions, but also in our apartment if not осущ...
Section: Articles about health
The depression not without reason is considered one their main troubles of our century: for scientific and technical progress, acceleration of rate of life and a surplus of information of people it is forced to pay with stresses, negative emotions and weakening of protective forces of an organism. As a result widely the states which are characterized by the increased uneasiness, falling of interest in life, spiritual and physical discomfort extend....
Section: Articles about health
Musicotherapy – a treatment method which caused and causes a set of a controversy concerning its efficiency. However the facts are relentless:...
Section: Articles about health
It is possible to find the extensive range of fruit and vegetables in modern shops. Russians already got used that on counters there is not only a seasonal domestic production, but the vegetables and fruit which are grown up in the countries with more comfortable conditions at all seasons of the year...
Section: Articles about health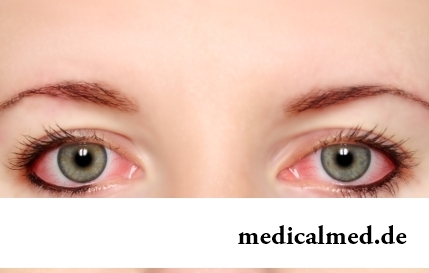
The sclera and mucous membrane of an eye are intensively supplied with blood vessels which problem - to saturate nervous tissues of body with nutrients and oxygen. In a normality vessels are almost not noticeable, however at their expansion (owing to thinning of walls) become visible, painting a sclera in red color. Quite often red eyes - the signal of any trouble in an organism caused as external irritants, allergens, and diseases which need in about...
Section: Articles about health
Venereal diseases in medicine are called the infections which are transmitted preferential sexually, now they are so...
Section: Articles about health
To look healthy and means well-groomed not only to be pleasant to people around, but also to feel strong, sure and taken place. Specialists in the field of cosmetology quite often note that whether not all women are able to look after skin...
Section: Articles about health
Olive oil – the product capable to make a powerful contribution to health of the person if it includes it in the diet. The rich vitamin composition of oil does it by a product number one from many diseases including from deadly. Only two tablespoons of oil from olives in day prevent emergence of diseases of vessels and heart, cancer, problems with digestion, presenilation, a depression and many other illnesses which treatment would demand a lot of time and forces. Let's consider on...
Section: Articles about health
Any of us is not insured from a heavy illness of the loved one. Happens and so that someone from family members becomes lying бо...
Section: Articles about health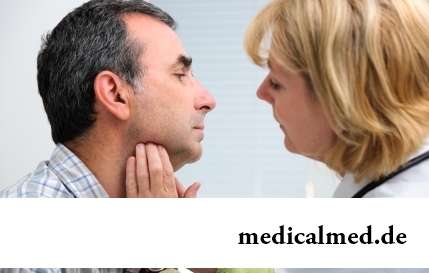
The endocrine system carries out extremely important role in a human body, practically all processes of life activity are regulated by it. Closed glands (hemadens) produce special biologically active agents – hormones which then o...
Section: Articles about health
Cold – a state known to everyone which is followed by cold, cough, high temperature, a pharyngalgia. Often the first that we begin to do in hope again to become healthy – to accept medicines which are not always harmless whereas it is easy to facilitate displays of a disease by means of natural means. They not only softly eliminate disease symptoms, but also enrich the weakened organism with useful substances. We present you 8 drinks which are successfully used for...
Section: Articles about health
Dogrose – one of the most widespread adornment and medicinal plants growing practically in all territory ours...
Section: Articles about health
Among a set of the perfumery and cosmetic goods which are released today the special group is made by the means containing antibacterial components. Such types of gels, shampoos, soaps, creams, lotions and other products are positioned by manufacturers as a panacea...
Section: Articles about health
For the city dweller the fitness is the most convenient sport. It is enough to acquire the subscription to the gym to get access to various apparatuses and an opportunity to train under the leadership of the experienced consultant. Many consider fitness the best way of maintenance of physical shape and receiving dynamic loads which the people occupied preferential with brainwork so need. Nevertheless, representations of most of consumers of similar services about специф...
Section: Articles about health
Obesity is called by a disease of 21 centuries, for the last 100 years by the number of the people suffering from excess body weight, considerably increased...
Section: Articles about health
Visit of doctors – business not the most pleasant, and many people do not hurry to undergo necessary planned inspections. Such behavior is extremely thoughtless and improvident. Our health is necessary not only to us: wellbeing of darlings, children, grandsons and престар...
Section: Articles about health
History of mankind contains several tens of epidemics whose emergence was compared by eyewitnesses and historians to doomsday. The most terrible of them claimed the lives of millions of people, having made even the whole people to the person of the earth. What they − the diseases striking terror? Whether it managed to the person to find treatment, or he is still powerless before forces of nature?...
Section: Articles about health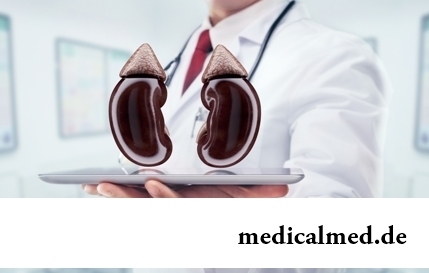
Kidneys perform the most important function of clarification of blood from those products of metabolic processes which cannot be used орг...
Section: Articles about health
One of the useful properties presented to the person by the nature is ability to feel fear. This ability is designed to signal about approach of a dangerous situation and to help to avoid in advance it to keep life. However if the fear is persuasive and not about...
Section: Articles about health
The state of health of the person depends on many factors. One of the most important is the constant, but not exhausting a physical activity. In the presence of various illnesses specialists often advise patients to do swimming which by right takes the leading place by efficiency of improvement, having at the same time a few contraindications. Today we will talk about the main directions of therapeutic impact of swimming on a human body....
Section: Articles about health
The majority of gynecologic diseases prove three main signs, each of which speaks about need to the visa...
Section: Articles about health
On the head of the person about one million hair follicles, or as they are called still, hair bulbs are located. At the time of the birth most of them is in the "sleeping" state, but within several weeks follicles become more active, and from them begin р...
Section: Articles about health
The list of stereotypes of which, apparently, all know strongly includes following: British surely eat porridge for breakfast. Perhaps, not all modern residents of Britain arrive quite so, but for those from them which continue to follow this tradition, it is possible to be glad sincerely: oat flakes are a product which regular use not only helps the person to keep force and beauty long. Porridge in a special way influences an organism, protecting it from seriousness...
Section: Articles about health


