





Meridia
Application instruction:
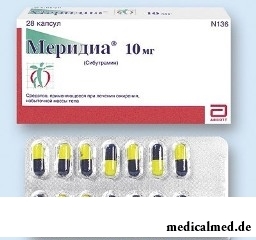 Meridia – the drug used for the supporting treatment of patients with alimentary obesity.
Meridia – the drug used for the supporting treatment of patients with alimentary obesity.
Form of release and structure
The Meridi is released in the form of capsules: firm gelatinous, with a blue lid and the case yellow (10 mg) or white (15 mg) colors, with a text (depending on a dose) "10" or "15"; contents of capsules – easily loose powder of almost white or white color (on 10 mg: on 7 pieces in blister strip packagings, on 2 packagings in a cardboard pack; on 14 pieces in blister strip packagings, on 1,2 or 6 packagings in a cardboard pack; on 15 mg – on 14 pieces in packagings, on 2 packagings in a cardboard pack).
Is a part of 1 capsule:
- Active agent: monohydrate of a hydrochloride of a sibutramin – 10 mg;
- Auxiliary components: sodium lauryl sulfate, microcrystallic cellulose, monohydrate of lactose, colloid silicon dioxide, magnesium stearate, gelatin, titanium dioxide (E171), indigotin (E132), gray ink, quinolinic yellow (E104 dye).
Indications to use
The Meridi is appointed at a maintenance therapy of patients with excess body weight in the presence of the following indications:
- Alimentary obesity with a body weight index from 30 kg/sq.m and more;
- Alimentary obesity with a body weight index from 27 kg/sq.m and more with other risk factors which are caused by excess body weight, such as lipid metabolism disturbance (dislipoproteinemiya) or a diabetes mellitus 2 types.
Contraindications
- Obesity, the having organic reasons;
- Glaucoma;
- The serious violations of food (established and known) in the form of nervous anorexia (exhaustion) or nervous bulimia (unlimited passion to food);
- Neurologic pathology (Giles de la Tourett's syndrome);
- Diseases of mental character;
- The established disturbances of cardiovascular system: chronic heart failure in a decompensation stage, coronary heart disease, arrhythmia, inborn heart diseases, tachycardia, occlusal diseases of peripheral arteries, cerebrovascular diseases (tranzitorny disturbances of cerebral circulation, a stroke);
- Heavy functional disturbances of a liver and kidneys;
- Increase in function of a thyroid gland (hyperthyroidism);
- Hormonal active tumor of adrenal glands (pheochromocytoma);
- Existence of inadequately controlled arterial hypertension (arterial pressure> of 145/90 mm hg);
- The established pharmacological, alcohol and drug addiction;
- High-quality increase in a prostate with formation of a residual urine (benign hyperplasia of a prostate);
- Simultaneous use of monoamine oxidase inhibitors, and also the period of 14 days after their cancellation (after reception of Meridia it is also necessary to sustain a two-week interval before reception of MAO inhibitors);
- Combination therapy with other medicines for decrease in weight or the drugs operating on the central nervous system (neuroleptics, antidepressants), the drugs used at sleep disorders (tryptophane) or mental disorders;
- Pregnancy and period of feeding by a breast;
- Age up to 18 years;
- Age of 65 years;
- Hypersensitivity to drug components.
Women of childbearing age during reception of Meridia need to use contraceptives.
Route of administration and dosage
The Meridi is accepted inside, without chewing and washing down with enough liquid (a glass of water), it is more preferable – in the morning. Capsules can be combined with meal or to accept on an empty stomach.
At the beginning of therapy appoint 10 mg of Meridia daily. In the absence of efficiency (criterion weight reduction less than on 2 kg in a month is), in case of good tolerance of drug, increase in a daily dose up to 15 mg is possible. At 15 mg of Meridia of patients which are poorly reacting to reception (criterion weight reduction less than on 4 kg in a month is) performing further therapy is inexpedient.
In case of the admission of administration of drug to accept a double dose, changing the usual mode of dosing, does not follow. At the patients who insufficiently are well reacting to therapy (decrease in body weight less than 5% of initial level in 3 months of therapy), the course should not proceed longer than 3 months. It is not necessary to continue treatment if against the background of further reception after the reached weight reduction of the patient puts on the weight of 3 kg and more.
Therapy duration (using 10 or 15 mg of Meridia) should not exceed 1 year (due to the lack of sufficient data on safety and efficiency of therapy throughout the period more than the specified term).
During treatment it is recommended to patients to change habits and a way of life so that after end of a course of therapy to provide preservation of the reached weight reduction (at non-compliance with these requirements inevitably repeated increase in body weight).
The doctor can change the dosing mode recommended in the instruction.
Side effects
Most often side effects develop for the first month of therapy. Their frequency and expressiveness weaken over time. In general, disturbances have reversible and not character (> 10% – are frequent, 1-10% – sometimes, <1% – is rare):
- Alimentary system: often – a lock, appetite loss; sometimes – an exacerbation of hemorrhoids, nausea;
- Cardiovascular system and blood (hemostasis, hemopoiesis): sometimes – heartbeat, tachycardia, increase in arterial pressure, a vazodilatation (erubescence with a caumesthesia), moderate increase in pulse (on 3-7 beats per minute) and moderate rise in arterial pressure at rest (on 1-3 mm hg); in some cases – more expressed increase in arterial pressure and increase of heart rate (clinically significant changes of pulse and arterial pressure are registered preferential in an initiation of treatment (in the first 1-2 months));
- Integuments: sometimes – perspiration;
- Nervous system and sense bodys: often – sleeplessness, dryness in a mouth; sometimes – concern, a headache, dizziness, change of taste, paresthesia (disturbance of sensitivity of skin).
In isolated cases at use of Meridia the following clinically significant disturbances are described:
- Diseases of kidneys in the form of acute intersticial nephrite, a mezangiokapillyarny glomerulonephritis;
- Tranzitorny increase in activity of liver enzymes;
- Shenleyna-Genokh's purpura;
- Thrombocytopenia;
- Convulsive attacks;
- Acute psychosis (at one patient with the schizoaffective disturbance which was presumably existing prior to administration of drug).
Special instructions
It is necessary to accept Meridia only with observance of accurately certain conditions and special precautionary measures. Before performing therapy it is necessary to consult with the specialist.
The Meridi is recommended to be accepted in cases when all actions for reduction of weight are ineffective (criterion decrease in body weight less than on 5 kg for 3 months is).
Therapy needs to be carried out only in a complex (including the change of a way of life and a diet necessary for preservation of the reached weight reduction after cancellation of drug treatment), observing recommendations of the specialist with practical experience of treatment of obesity.
The patients accepting Meridia need to control the arterial pressure and heart rate. For 2 first months of treatment it is necessary to control these parameters each 14 days, then once a month. At arterial hypertension (arterial pressure over 145/90 mm hg) control of these parameters, if necessary, needs to be carried out more often and especially carefully. Therapy should be interrupted in cases if at repeated measurement arterial pressure exceeds 145/90 mm hg twice.
It is necessary to be careful at a combination therapy with the medicines increasing QT interval (sertindoly, terfenadiny, astemizoly, Amiodaronum, quinidine, flekainidy, propafenony, meksiletiny, sotaloly, Pimozidum, tsizapridy, tricyclic antidepressants), and at states which are capable to lead to increase in an interval of QT (for example, at a hypopotassemia and a hypomagnesiemia).
When carrying out regular medical control of a condition of accepting Meridia of the patient special attention needs to be paid on thorax pain, progressing диспноэ and hypostases standing (communication between administration of drug and development of primary pulmonary hypertensia is not established).
It is also necessary to be careful in the presence of the following diseases / states:
- Epilepsy;
- Functional disturbances of a liver of average and easy severity (because of possible increase in concentration of a sibutramin in a blood plasma);
- Functional disturbances of kidneys of average and easy severity (because of removal by kidneys of inactive metabolites of drug);
- Anamnestic data on motor or verbal tics (uncontrollable spontaneous muscular contractions, and also disturbances of an articulation);
- Simultaneous use with the drugs promoting increase in arterial pressure and heart rate (including the medicines applied at cough, an allergy and cold).
At cancellation of Meridia development of disturbances in the form of a headache and increase in appetite is in rare instances possible; there are no data on development of an abstinence syndrome, disturbances of mood or a withdrawal.
Because of need of observance of a diet to take alcohol during therapy it is not recommended.
The drugs operating on the central nervous system can limit memory, intellectual activity, speed of reactions. In this regard drivers of vehicles and patients whose profession is connected with the increased concentration of attention need to be careful at purpose of Meridia.
Medicinal interaction
At a combination therapy of Meridia with some drugs there can be following effects:
- The medicines inhibiting activity of CYP3A4 enzyme (тролеандомицин, кетоконазол, erythromycin, cyclosporine): increase in concentration in plasma of metabolites of a sibutramin, clinically insignificant lengthening of an interval of QT (on 9,5 ms), increase in heart rate (on 2,5 beats per minute);
- Rifampicin, dexamethasone, Phenytoinum, phenobarbital, carbamazepine, antibiotic drugs of group of macroleads: acceleration of metabolism of a sibutramin;
- The drugs increasing serotonin neurotransmitter level in a blood plasma (dihydroergotamine, суматриптан, selective serotonin reuptake inhibitors, the strong anesthetizing medicines (pethidine, pentazocine, fentanyl), antibechic drugs (including dextromethorphan)): increase in risk of development of a serotoninovy syndrome.
Does not render on effect of hormonal contraceptive drugs of Meridia of influence.
The provided data on medicinal interaction belong to the drugs used during the short period.
At a concomitant use of Meridia with alcohol strengthening of negative effect of the last was not noted. However alcohol is not combined with the dietary actions recommended at drug use.
Terms and storage conditions
To store in the place, dry, unavailable to children, at a temperature up to 25 °C.
Period of validity – 3 years.
The 74-year-old resident of Australia James Harrison became blood donor about 1000 times. It has a rare blood group which antibodies help to survive the newborn with a severe form of anemia. Thus, the Australian saved about two million children.

All of us, unfortunately, should face flu nearly an every year. It would seem, so frequent disease has to be study...
Section: Articles about health
From the failure of work of immune system which is shown in the form of an allergy, statistically, more than 40% of the population of the globe suffer. In most cases pathological reactions cause the substances which are contained in food stuffs, hair of animals, medicines...
Section: Articles about health
The nature does not stand stagnation and monotony. It is known that tissues of a human body atrophy if do not receive necessary loadings. It fully belongs also to a cerebral cortex: when it is not given full-time job, it begins to function worse. As a result memory decreases, the person becomes less bright, acquires information more slowly, hardly switches from one thought to another. There are problems at work, difficulties with communication and career development. These it is unpleasant...
Section: Articles about health
Sugar - the digestible refined product which is not of special value for an organism of the modern person. Use...
Section: Articles about health
Bulimia and anorexia, are heavy deviations of a feeding behavior, become a cause of death of patients much more often than all other nervous breakdowns combined. In 60% of cases two illnesses accompany each other: patients feel horror before danger on...
Section: Articles about health
Visit of doctors – business not the most pleasant, and many people do not hurry to undergo necessary planned inspections. Such behavior is extremely thoughtless and improvident. Our health is necessary not only to us: wellbeing of darlings, children, grandsons and aged parents directly depends on as far as we are vigorous and able-bodied. Therefore in time to be inspected – a duty of any modern person. Specialists consider that 7 regular surveys and di are especially necessary for women...
Section: Articles about health
Neurosis is called pathology of a nervous system at which deviations in functioning of the highest nervous processes are observed. Nye...
Section: Articles about health
Season of activity of viral infections in the heat. Everyone can get sick, but probability of this unpleasant event it is possible and it is necessary to minimize. There is a number of rules, following to which will help or to avoid absolutely infection with flu or a SARS, or to have an illness...
Section: Articles about health
Producers of milk mixes for children assure: mixes are ideally balanced and adapted for needs of babies. If mother should raise artificially the kid owing to serious problems with health, to do nothing – it is necessary to feed with substitutes of milk. However pediatricians note that not seldom women without good reasons refuse feeding of the child a breast and pass to milk mixes. Common causes of such decision – the aspiration to leave quicker...
Section: Articles about health
The modern person not always manages to find housing in the environmentally friendly region and such work which would not do harm здо...
Section: Articles about health
History of use of an anesthesia during operations contains more than 160 years. Annually in the world hundreds of thousands of surgical interventions during which to patients the substances immersing them in a dream and saving from pain are entered are carried out. Using an anesthesia to these...
Section: Articles about health
For most of the working people the problem of having a snack is particularly acute enough. Sooner or later there is a question: what can be eaten quickly between a breakfast and a lunch or a lunch and leaving from service so that to receive necessary power feed, but not to overload an organism with harmful components or excess calories? We bring to your attention the list of products which quite conform to these requirements....
Section: Articles about health
Obesity is called by a disease of 21 centuries, for the last 100 years by the number of the people suffering from excess body weight, considerably increased...
Section: Articles about health
The main role in development of a peptic ulcer of a stomach and duodenum the bacterium Helikobakter plays pilor. Activity and the strengthened reproduction of this microorganism lead to weakening of protection of mucous membranes and their erosive damage. Manifestations not...
Section: Articles about health
About 20% of the population of our planet have a hypertension (permanent increase in arterial pressure). This disease has an adverse effect on the standard of living, reduces working capacity, and in the absence of systematic treatment threatens with such complications as a myocardial infarction, a stroke and other heavy illnesses which can result in disability or sudden death. Most of patients for maintenance of pressure at more or less acceptable level accept appointed doctors лекарст...
Section: Articles about health
The thought that the mass of their body is too big at least once in life visits from 80 to 95% of women. Many...
Section: Articles about health
What will only not be thought up by persons interested to have a beautiful figure. Here the last innovation – for weight loss needs to be eaten greasy food. Let's understand whether there is at a fatty diet common sense....
Section: Slideshow
Many parents of children at the age of 2-4 years face excessively whimsical behavior of the child. The kid exhausts constant crying and whims not only the parents, but also himself. In what the reasons of children's whims. And how to fight with them?...
Section: Slideshow
Mushrooms - the surprising inhabitants of our planet having a set of wonderful qualities. Thanks to one of them, a mold mushroom of Penici...
Section: Articles about health
At this plant there are a lot of names: tuberiferous sunflower, Jerusalem artichoke, solar root, earth pear. Contrary to popular belief, it is not an exotic plant at all. The wild girasol grows in a midland of Russia practically everywhere: at the edges of roads...
Section: Articles about health
About influence of fasting days on an organism it is told much – both about advantages, and about shortcomings. It is considered that fasting day in the form of a short-term monodiet is useful, promoting effective removal of slags from an organism whereas irregular, excessively long, spontaneous fasting days lead only to deterioration in health. How to derive benefit from the sparing diet and not to do much harm to itself? Let's consider the main advantages and shortcomings of fasting days and their influence on an org...
Section: Articles about health
(Xerostomia) many people consider feeling of a xerostomia small and easily removable inconvenience. This delusion...
Section: Articles about health
Bathing in broths of medical flowers and plants (phytobathtub) was eurysynusic since Cleopatra who is a good judge in all that concerns beauty and health. And today phytobathtubs is the simple and available means allowing not only to remove nervous N...
Section: Articles about health
Memory is an ability of the central nervous system to fix, keep and as necessary to reproduce information on knowledge or skills received by the person or an animal during life. The mechanism of this process is up to the end not studied....
Section: Articles about health
Turnip, radish, horse-radish – once these and other products enjoyed wide popularity at our ancestors, being not only food, N...
Section: Articles about health
Coffee - the tonic loved by many for the invigorating aroma and deep taste. Having the stimulating effect, coffee increases working capacity, promotes concentration of attention, fights against drowsiness and improves mood. Statistically, about 30% of inhabitants...
Section: Articles about health
The state of health of the person in many respects depends on food. The organism will well function if during food it receive only useful substances, necessary vitamins and microelements. In this case there will be no problems with digestion, with excess weight, and intellectual and physical activity will remain at the high level....
Section: Articles about health
