Kombivir
Application instruction:
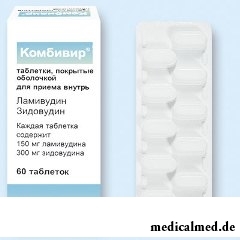 Kombivir – the antiviral combined medicine active concerning the human immunodeficiency virus (HIV).
Kombivir – the antiviral combined medicine active concerning the human immunodeficiency virus (HIV).
Form of release and structure
Dosage form of Kombivir – a tablet, film coated: oval, white or almost white color, on one of the parties the text divided by risky – "GXFC3" (on 10 pieces in blisters PVC, on 6 blisters in a cardboard pack Is scarlet/) is engraved.
Contain in 1 tablet:
- Active ingredients: ламивудин – 150 mg, a zidovudine – 300 mg;
- Auxiliary components: anhydrous colloid silicon, microcrystallic cellulose, sodium carboxymethylstarch (type A), magnesium stearate;
- Film cover: опадрай white (titanium dioxide, gipromelloz, polysorbate 80, macrogoal 400).
Indications to use
Kombivir apply to therapy of HIV infection at children (weighing not less than 14 kg) and adult.
Contraindications
- Heavy anemia (the indicator of hemoglobin is lower than 4,65 mmol/l or 7,5 g/dl);
- Heavy neutropenia (the quantity of neutrophils are less 0,75×109/л);
- Body weight at children less than 14 kg;
- Hypersensitivity to a zidovudine, a lamivudin or any of medicine components.
The zidovudine reduces the level of transfer of HIV from mother to the child at Kombivir's reception by pregnant women, with the subsequent therapy of the newborn. However there are no similar data on a lamivudin, its safety of use at pregnancy is not established. Also there are no data on the combined use of a zidovudine and a lamivudin. Therefore to pregnant women, especially in the I trimester, drug is recommended to use, only if the expected advantage for mother is higher than probable risk for a fruit.
As ламивудин, the zidovudine and a human immunodeficiency virus get into breast milk, to HIV-positive women who are treated by Kombivir, under any conditions it is impossible to nurse the child.
At abnormal liver functions of heavy degree and at a renal failure with the clearance of creatinine (CC) of ≤50 ml/min. it is recommended to use separately drugs of a zidovudine and lamivudin.
At therapy of elderly patients it is necessary to observe extra care taking into account age changes, such as a renal failure or changes of hematologic indicators.
Route of administration and dosage
Specialists with experience of therapy of HIV-positive patients have to carry out treatment by Kombivir.
Pill Kombivir is taken inside, swallowing entirely, irrespective of meal. For patients who cannot swallow a tablet entirely it should be crushed and added to a small amount of liquid or food and to accept immediately right after crushing.
The recommended dosing mode for adults and children depending on body weight:
- ≥30 kg – 1 таб. 2 times/days;
- 21-30 kg – 1/2 таб. in the morning and 1 таб. in the evening;
- 14-21 kg – 1/2 таб. 2 times/days.
If it is necessary to reduce Kombivir's dose, to cancel ламивудин or a zidovudine, or to lower a dose of one of these components, it is recommended to apply monodrugs of a lamivudin (Epivir – tablets or solution to intake) and a zidovudine (Retrovir – solution to intake or the capsule).
At anemia (hemoglobin of ≤5,59 mmol/l (9 g/dl) or neutropenias (neutrophils ≤1,0 x 109/l) dose adjustment of a zidovudine therefore at such indicators it is necessary to use monodrugs of a zidovudine and lamivudin is perhaps necessary.
Side effects
For today there are no data on the additive toxicity of a zidovudine and lamivudin therefore Kombivir can cause the side effects characteristic of each of these components separately.
Side effects of a lamivudin:
- Hemopoietic and lymphatic system: infrequently – anemia, thrombocytopenia, a neutropenia; very seldom – a true erythrocyte aplasia;
- Metabolism and food: often – a giperlaktatemiya; seldom – lactic acidosis, accumulation/redistribution of lipidic fabric (frequency depends on various factors, including a specific anti-retrovirus combination of drugs);
- Nervous system: often – a headache; very seldom – paresthesias, there are messages on peripheral neuropathy, but its communication with reception of a lamivudin is unknown;
- Digestive Tract (DT): often – pain in epigastriums, nausea, vomiting, diarrhea; seldom – increase in level of serumal amylase, pancreatitis (there are no reliable data about communication with therapy lamivudiny);
- Gepatobiliarny system: infrequently – tranzitorny increase in alaninaminotranspherase (ALT) and aspartate aminotransferase (nuclear heating plant);
- Skin and hypodermic fatty tissue: often – an alopecia, rash;
- Musculoskeletal system and connecting fabric: often – muscular disturbances, an arthralgia; seldom – рабдомиолиз;
- General and local reactions: often – a febricula, fatigue, fever.
Side effects of a zidovudine:
- Hemopoietic and lymphatic system: often – a leukopenia, a neutropenia and anemia (perhaps, hemotransfusion will be required); infrequently – a pancytopenia (with a marrow hypoplasia) and thrombocytopenia; seldom – a true erythrocyte aplasia; very seldom – aplastic anemia;
- Metabolism and food: often – a giperlaktatemiya; seldom – anorexia, lactic acidosis, accumulation/redistribution of lipidic fabric (frequency depends on various factors, including a specific anti-retrovirus combination of drugs);
- Mentality: seldom – an alarming state, a depression;
- Nervous system: very often – a headache; often – dizziness; seldom – paresthesias, drowsiness, sleeplessness, spasms, decrease in intellectual activity;
- Cardiovascular system: seldom – a cardiomyopathy;
- Respiratory system, bodies of a thorax and mediastinum: infrequently – an asthma; seldom – cough;
- GIT: very often – nausea; often – an abdominal pain, vomiting, diarrhea; infrequently – a meteorism; seldom – a food faddism, pigmentation of a mucous oral cavity, dyspepsia, pancreatitis;
- Gepatobiliarny system: often – increase in levels of bilirubin and liver enzymes; seldom – damages of a liver, such as the expressed hepatomegalia with a steatosis;
- Skin and hypodermic fatty tissue: infrequently – an itch, rash; seldom – a xanthopathy and nails, a small tortoiseshell, perspiration;
- Musculoskeletal system and connecting fabric: often – a mialgiya; infrequently – a myopathy;
- Kidneys and urinary tract: seldom – a frequent urination;
- Reproductive system and mammary glands: seldom – a gynecomastia;
- General and local reactions: often – a febricula; infrequently – an adynamy, fever, a generalized pain syndrome; seldom – a stethalgia, a fever, a grippopodobny syndrome.
Special instructions
In case of need selection of a dose use of separate drugs of a zidovudine and lamivudin is individually recommended. The attending physician has to be guided by the application instruction of these medicines.
As Kombivir's use or other anti-retrovirus drugs does not exclude development in patients of opportunistic infections and other complications of HIV infection, patients should be under regular observation of the medical personnel having experience of therapy of HIV infection.
There are no data on influence of a zidovudine and lamivudin on female fertility.
Therapy by Kombivir, as well as other anti-retrovirus drugs, does not prevent a possibility of transfer of HIV at transfusion of the infected blood or sexual contacts therefore it is necessary to observe the appropriate measures of precaution.
Special instructions on Kombivir's use at various states / diseases:
- Hematologic disturbances: reception of a zidovudine can provoke development of a neutropenia, anemia and a leukopenia (usually secondary owing to a neutropenia); more often such effects are observed in case of purpose of high doses of a zidovudine (1,2-1,5 g/days) at late stages of a disease at decrease in a marrowy reserve prior to therapy therefore at the patients receiving Kombivir it is necessary to carry out thorough control of indicators of blood. As such hematologic pathologies usually develop 4-6 weeks later from an initiation of treatment, patients with the developed clinical picture of HIV infection are recommended to control blood indicators at least 1 time in 2 weeks in the first three months of therapy, then – at least 1 time a month. At HIV infection at an early stage from system of blood side effects are noted seldom therefore the general blood test is admissible to be done, being guided by health of patients, 1 times in 1-3 months. In case of development of a miyelosupressiya or heavy anemia in the course of therapy by Kombivir, and also at patients with the previous oppression of marrow (hemoglobin is lower than 5,59 mmol/l (9 g/dl) or neutrophils less 1,0×109/л) perhaps it will be necessary to select specially a zidovudine dose and as in drug Kombivir separately it cannot be made, it is recommended to use monodrugs of a zidovudine and lamivudin;
- Pancreatitis: rare episodes of development of pancreatitis in the patients accepting a zidovudine and ламивудин are described, but whether the complication is established authentically, connected with administration of drugs or with a basic disease – HIV infection. Therapy should be stopped immediately in case of the clinical signs or these laboratory researches indicating development of pancreatitis (nausea, vomiting, pain in epigastric area, increase in level of biochemical markers), to an exception of suspicion of pancreatitis;
- Acidosis lactic / the expressed hepatomegalia with a steatosis: there are data about rare, but with probability of a lethal outcome, cases of lactic acidosis and the expressed hepatomegalia with fatty dystrophy of a liver (most of them is registered at women). Clinical symptoms of lactic acidosis are: general weakness, loss of appetite and sudden inexplicable loss of weight, gastrointestinal disturbances, increase of breath and asthma. It is necessary to suspend Kombivir's use if at the patient clinical and laboratory symptoms of acidosis lactic or hepatotoxics (including a hepatomegalia and a steatosis are observed even if levels of transaminases are not increased). With risk factors of damage of a liver drug needs to be taken with caution;
- Redistribution of a hypodermic fatty tissue: at some patients the following symptoms can be observed (separately or in a complex): accumulation/redistribution of lipidic fabric, including a dorsotservikalny adiposity at the neck basis – "a buffalo hump", the central type of obesity, increase in mammary glands, thinning of a front hypodermic and fatty layer and reduction of a lipidic layer on extremities, increase in levels of glucose and serumal lipids in blood. At the same time the syndrome of a lipodystrophy is caused by a multifactorial etiology; for example, advanced age, a stage of HIV infection and duration of a course of anti-retrovirus therapy are perhaps synergistic, and play an important role in accumulation/redistribution of lipids owing to Kombivir's use. Now the remote effects of noted side effects are unknown. Disturbances of a lipometabolism it is necessary to treat depending on their clinical manifestations. It is necessary to determine levels of glucose and lipids in blood serum, and also to perform clinical examination of patients which has to include assessment of physical signs of redistribution of lipidic fabric;
- Immunity recovery syndrome: at the beginning of therapy by anti-retrovirus drugs of patients with HIV infection at a heavy immunodeficiency against the background of a residual or symptomless opportunistic infection the aggravation of inflammatory process is probable, it can seriously worsen a state or aggravate symptomatology. Usually such effects are observed for the first weeks or months from the beginning of anti-retrovirus treatment, the most significant of them – the generalized and/or localized (focal) mikobakterialny defeat, a Cytomegaloviral retinitis and a pneumocystosis. Any signs of inflammatory processes should be revealed immediately and in case of need at once to start therapy;
- The accompanying viral hepatitis In: Kombivir is recommended to apply with care at the dekompensirovanny cirrhosis caused by chronic hepatitis B as the exacerbation of hepatitis in case of cancellation of a lamivudin in rare instances can develop. Periodic monitoring of work of a liver and markers of replication of a virus of hepatitis B is necessary;
- The accompanying viral hepatitis With: at a concomitant use of a zidovudine and a ribavirin aggravation of anemia (the mechanism of development of effect is not found out) therefore the combined use of a ribavirin and Kombivir it is not recommended, especially in the presence of data in the anamnesis about a zidovudine - the induced anemia was observed. It is necessary to consider the possibility of cancellation of a zidovudine as a result of change of the mode of anti-retrovirus therapy;
- Driving of motor transport and control of difficult mechanisms: special researches of influence of a zidovudine and lamivudin for the period of reaction and speed of psychomotor reaction were not conducted, proceeding from pharmacological properties of these drugs such influence is improbable, but it is necessary to consider a clinical condition of the patient and the nature of side effects of a zidovudine and lamivudin.
Medicinal interaction
Kombivir are a part of drug a zidovudine and ламивудин therefore he can enter the interactions characteristic of any of its components.
Only small part of a lamivudin participates in a metabolism and contacts proteins of plasma. Almost completely drug in an invariable look is removed by kidneys therefore the probability of metabolic interactions is small. Nevertheless it is necessary to consider the following interactions with lamivudiny:
- The medicines which are removed with the help a cation - transport system – interaction with lamivudiny because of use of one way of removal is possible;
- Co-trimoxazole (160 mg of Trimethoprimum + 800 mg of sulfamethoxazole) – for 40% increases concentration in plasma of a lamivudin (reception in therapeutic doses). At normal function of kidneys dose adjustment of a lamivudin is not necessary, patients with a renal failure have at the same time Co-trimoxazole and Kombivir are applied with care. ламивудин does not exert impact on pharmacokinetics of sulfamethoxazole or Trimethoprimum. The effect of combined use of drugs for therapy of a toxoplasmosis and a pneumocystosis was not studied;
- Zaltsitabin – is not recommended to use in a combination with Kombivir as ламивудин his intracellular phosphorylation can oppress.
The zidovudine contacts proteins of plasma slightly, preferential it eliminirutsya by means of hepatic metabolism to an inactive glucuronide.
Interactions with a zidovudine:
- Atovakvon – a zidovudine does not influence its pharmacokinetics, but атоваквон reduces extent of metabolism of a zidovudine to its glucuronide (AUC of a zidovudine increases in an equilibrium state for 33%, and Cmax in plasma of a glucuronide decreases by 19%). At use of a zidovudine from 500-600 mg/days in a complex with a 3-week course of therapy of an acute pneumocystosis atovakvony increase in frequency of the side effects connected with the increased concentration in zidovudine plasma is improbable. In case of need more long course of joint therapy it is necessary to watch a clinical condition of the patient carefully;
- Klaritromitsin – reduces absorption of a zidovudine (not less than 2 hours are required to observe an interval between receptions of a klaritromitsin and a zidovudine);
- Lamivudin – raises time of influence of a zidovudine for 13%, increases by 28% it Cmax in plasma, but at the same time considerably does not change the general exposure of a zidovudine (AUC); the zidovudine does not influence pharmacokinetics of a lamivudin;
- Phenytoinum – decrease in blood of concentration of Phenytoinum was in certain cases noted, and in a separate case concentration of Phenytoinum increased (therefore, at simultaneous use of Kombivir and Phenytoinum control of concentration of Phenytoinum in blood is necessary);
- Probenetsid – can increase average T1/2 of a zidovudine and AUC because of oppression of formation of a glucuronide, to reduce renal excretion of a glucuronide and, perhaps, a zidovudine;
- Rifampicin – perhaps reduces AUC of a zidovudine by 48±34%, but clinical value of this effect is unknown;
- Stavudin – the inhibition of process of intracellular phosphorylation of a stavudin a zidovudine is possible at their concomitant use therefore such combination is not recommended;
- Acetylsalicylic acid, morphine, methadone, codeine, indometacin, ketoprofen, oxazepam, lorazepam, Naproxenum, Cimetidinum, Clofibratum, изопринозин – can break metabolism of a zidovudine because of competitive inhibition of a glyukuronization or direct suppression of microsomal metabolism. Before purpose of these drugs along with Kombivir, especially for long therapy, it is necessary to estimate effects of possible medicinal interactions;
- Potentially nefrotoksichny or myelosuppressive drugs (especially at therapy of acute states): pentamidine (at system introduction), dapsone, Pyrimethaminum, co-trimoxazole, Amphotericinum, флуцитозин, ганцикловир, interferon, Vincristinum, vinblastine, doxorubicine – can increase risk of side effects of a zidovudine (in case of their co-administration with Kombivir careful control of function of kidneys and hematologic indicators is necessary, if necessary it is required to lower a dose of one or several drugs).
As, despite Kombivir's reception, at some patients opportunistic infections can develop, perhaps their prevention will require purpose of an additional antimicrobic course of treatment. Co-trimoxazole, Pyrimethaminum, pentamidine (aerosol) and an acyclovir as by results of clinical tests the expressed increase in frequency of side effects of a zidovudine at simultaneous use with these drugs is absent are for this purpose recommended.
Terms and storage conditions
To store in the place, unavailable to children, at a temperature not above 30 °C.
Period of validity – 2 years.
Having fallen from a donkey, you more likely will kill yourself, than having fallen from a horse. Only do not try to disprove this statement.

Traveling all over the world, many try to try the most exotic dishes of national cuisines. Exists even so-called died away...
Section: Articles about health
Iodine - one of thirty most important microelements in our organism. The main role of iodine consists in synthesis of thyroid hormones of a thyroid gland - the substances which are responsible for the majority of exchange processes of an organism. It is known that thyroid hormones consist...
Section: Articles about health
The dietology, as well as other sciences, does not stand still. Food stuffs are exposed to comprehensive study, and scientists obtain new information on their properties and influence on a human body. Unfortunately, this reasonable and natural process gives unpleasant side effect from time to time: some types of food periodically declare "harmful" or even "deadly" without the bases, sufficient on that....
Section: Articles about health
Life does not indulge the modern woman special emotional comfort and carelessness. Fatigue, troubles at work, misunderstanding...
Section: Articles about health
The business lady, the become mother, it is necessary to solve an array of problems. But of them is main: how to combine the beloved child and work? What traps trap the working mother and how she needs to behave?...
Section: Slideshow
The winter swimming in open reservoirs called in our country by "winter swimming" – officially recognized sport and one of the most extreme ways of a hardening of an organism. This occupation has an old story and adherents in many countries. The international competitions in winter heats on open water, and every two years – the World Cup are annually held. Despite huge popularity and the proved advantage for health, winter swimming is still surrounded with hardy delusions. Ра...
Section: Articles about health
The body of the person almost for 60% consists of water. It is so important for normal functioning of an organism that loss of all is ponut...
Section: Articles about health
All like to sing. Small children with pleasure are engaged in a vocal, not especially thinking of hit in a melody. Adults most often hesitate, being afraid to show lack of talents in this area, and it is vain: singing is very useful for health....
Section: Articles about health
Sometimes it seems that modern society was divided into two camps: representatives of the first are sure that only the woman has to be responsible for contraception, representatives of the second, respectively, are sure that it is destiny of men. Meanwhile the question of contraception has very many aspects – both psychological, and legal and, of course, medical....
Section: Articles about health
The words "disease" and "patient" not without reason come from one root – "pain". As a rule, symptoms of illnesses thoroughly spoil the patient...
Section: Articles about health
For most of the working people the problem of having a snack is particularly acute enough. Sooner or later there is a question: what can be eaten quickly between a breakfast and a lunch or a lunch and leaving from service so that to receive necessary power feed, but not an overload...
Section: Articles about health
For many women the word "fat" sounds as a sentence. In aspiration to an ideal figure they try to exclude, first of all, from the menu all dishes containing fats without having at the same time a clear idea of a role of these substances in exchange processes, and of effects for health with which food restrictions of this sort are fraught. For what the human body needs fats and as their deficit in a diet is shown, we also will try to find out....
Section: Articles about health
Shops of household appliances offer us the huge choice of various devices for the house. Whether there are among this abundance devices which...
Section: Articles about health
The saying "the rich do not know how the other half lives" is known to all. In a broad sense it is that we can not always understand the person whose features of a state are unknown to us. If with physiological characters of diseases the situation is more or less clearly (having noticed and...
Section: Articles about health
The next flu epidemic leads to the next panic, from year to year we give in on these manipulations: professionally alarming voice of the announcer in news, reports with calculation of the died patients, an interview with people in white dressing gowns and advertizing of anti-influenza means of different degree of inefficiency. All this reminds the Hollywood movies of epidemics threatening to destroy our planet. However, there is also one more similarity to cinema: everything comes to an end well. So, how to deal with the events, not in...
Section: Articles about health
Childbirth is the most important event in life of each woman. We are women we give birth to the new little man on this light. Now...
Section: Articles about health
The trophic ulcer is not an independent disease. This heavy complication arising owing to a thermal injury (a burn or a frostbite), chronic pathologies of arteries or veins of the lower extremities, a diabetes mellitus, and also some defeats of a soyeda...
Section: Articles about health
Water with a lemon - idle time in preparation drink which supporters of a healthy lifestyle already managed to appreciate. Used in a warm look and on an empty stomach, it is one of the most useful prophylactics allowing to prevent tens of diseases and just to raise an organism tone. Especially effectively to use warm water with lemon juice after a serious illness, during a season of the colds, and also to children, old men and pregnant women which do not have contraindications...
Section: Articles about health
The metabolism at each person proceeds in own way. However dependence between the speed of this process and disposal from superfluous in...
Section: Articles about health
Contrary to popular belief, the multiple sclerosis (MS) is not connected neither with sclerous changes of walls of vessels, nor with age forgetfulness and problems with concentration of attention. This disease has the autoimmune nature. Pathological process of a vyrazh...
Section: Articles about health
The fatigue, sleep debt, disturbances of food, bad mood, vagaries of the weather – all these circumstances badly affect our appearance. Especially the person suffers: skin becomes flabby, loses healthy color, becomes covered by wrinkles, zones of hypostases and dark circles under eyes appear. It is not always possible to be saved from influence of aggressive factors, but we are quite able to minimize its effects. For this purpose usually apply the cosmetics and procedures helping увлаж...
Section: Articles about health
Maternal milk is the best food for the newborn. It is the unique natural product containing optimum set...
Section: Articles about health
Eyes – one of the most vulnerable areas on a face therefore age changes concern them first of all. Whether it is possible to keep look youth for many years and what procedures are offered for achievement of this purpose by cosmetologists? And maybe, only thing of a vari...
Section: Articles about health
Coffee - the tonic loved by many for the invigorating aroma and deep taste. Having the stimulating effect, coffee increases working capacity, promotes concentration of attention, fights against drowsiness and improves mood. Statistically, about 30% of inhabitants of the planet regularly use coffee, from them more than 8% are "coffee-achievers" - the persons using more than 3 cups of drink a day....
Section: Articles about health
One of the major chemical processes happening in a human body are oxidation reactions. They go with participation of fats...
Section: Articles about health
For the time being the perspective of heart diseases seems to most of people remote and foggy. But sooner or later practically each adult faces extremely unpleasant feelings: sudden stethalgia. To be consoled at this time in a thought of t...
Section: Articles about health
The chia plant, or the Spanish sage, is from South America. The indigenous people of the continent since ancient times used its seeds in food: small, but very nutritious kernels, in a form the reminding fasolina. Indians knew about useful properties of seeds of a chia, and applied them to maintenance of vitality and increase in endurance before serious exercise stresses....
Section: Articles about health